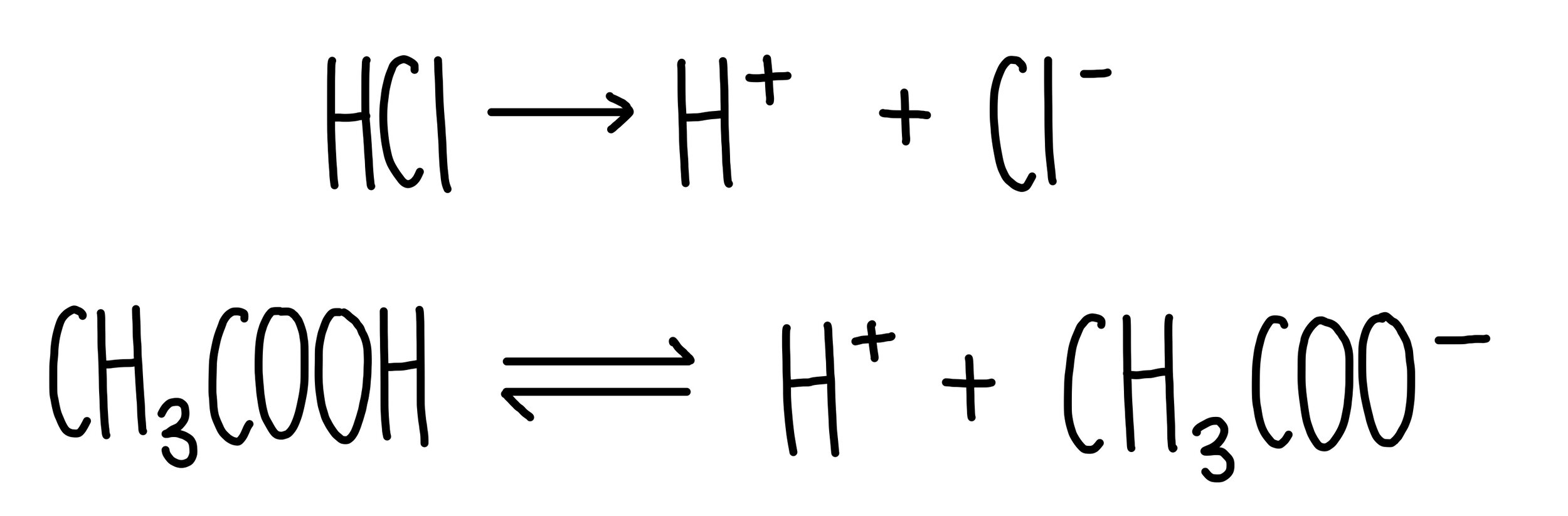Acids
Acids, alkalis, and bases
Acids are substances which release hydrogen ions (H+) in solution. Strong acids completely dissociate into hydrogen ions, which means that all molecules will break apart to release hydrogen ions. Strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4) and nitric acid (HNO3). Weak acids only partially dissociate into hydrogen ions, which means that only some molecules will ionise into H+ in solution.
Equations showing the dissociation of strong acids are represented with a single arrow whereas weak acid dissociation is represented with a double-headed arrow. Weak acids include the carboxylic acids such as ethanoic acid (CH3COOH).
Alkalis are substances which release hydroxide ions (OH-) in solution. Strong alkalis include sodium hydroxide (NaOH) and potassium hydroxide (KOH). A weak alkali is ammonium hydroxide (NH4OH).
A base is something which can neutralise an acid. For example, metal oxides and metal carbonates are bases since they can react with alkalis and neutralise them. Alkalis are just a special type of base – it’s a base which is soluble in water. This means that all alkalis are bases, but not all bases are alkalis.
Acids react with three types of bases (alkalis, metal oxides and metal carbonates) as shown in the equations below:
These are all examples of neutralisation reactions – the reaction between an acid and a base to form a salt and water. Remember that a salt is simply what is formed when they hydrogen in an acid is replaced with the metal ion. Neutralisation reactions can be simplified down to the ionic equation:
Titration method
We use titrations to work out the concentration of an acid or an alkali.
To carry out a titration, put the acid in a conical flask using a pipette. Then take a solution of alkali of a known concentration and use it to fill a burette. You also need to add an indicator to the conical flask – this is something that will change colour when neutralisation has occurred.
Record the initial volume of alkali in the burette then add it to the acid in the conical flask very slowly. The conical flask should be swirled regularly to ensure the acid and alkali are mixing properly. Keep adding the alkali dropwise until the end point is reached, which is when the indicator has changed colour. Record the final volume of alkali in the burette and subtract from the initial volume to work out how much was needed for neutralisation. This is your titre.
You’ll need to make sure you’ve not botched the titration somehow by repeating a couple more times to see if you get concordant results. Concordant results are titres that are within 0.1 cm3 of each other. Make sure that you discard any data that is not concordant and calculate a mean titre. Now that we know the concentration and volume of the alkali needed for neutralising our acid, we can calculate the concentration of the acid (see calculations below).
Note that you can carry out a titration the other way round – with an alkali of unknown concentration in your conical flask and an acid of known concentration in the burette.
Indicators
Indicators are dyes that change colour depending on the pH of the solution. For a titration, we need to use indicators that change colour over a very narrow pH range so that we achieve an accurate end-point. The indicators used for titrations include phenolphthalein and methyl orange. To better see the colour change, it’s a good idea to place a white tile under the conical flask.
Phenolphthalein: turns pink to colourless when adding acid to alkali
Methyl orange: turns yellow to red when adding acid to alkali
Titration calculations
So we’ve carried out our imaginary titration, got three concordant titres (because we’re bossing it) and have calculated a mean titre of 32.6 cm3 for the alkali that we added. Let’s say the alkali we used was 0.5 mol dm-3 sodium hydroxide and the acid we were titrating it against was sulfuric acid. If we added 25 cm3 of the acid to the conical flask, what’s the concentration of the sulfuric acid?
The first thing we need to do is to write a balanced symbol equation. In the exam this may be given to you already, but not always.
From the balanced symbol equation, I can see that there’s a 2:1 ratio between the alkali (sodium hydroxide) and the acid (sulfuric acid).
Now, because we know the concentration and volume of the alkali, we can work out its concentration using the moles = concentration x volume equation. Don’t forget to convert the volume in cm3 to dm3 by dividing it by 1000 before popping it into your equation!
32.6 cm3 / 1000 = 0.0326 dm3
0.5 x 0.0326 = 0.0163 mol of NaOH
From the 2:1 ratio, I know that I have twice the number of moles of alkali compared to acid. So to work out the moles of acid, I’m just going to divide our answer by 2.
= 8.15 x 10-3 mol H2SO4
Now I have the volume and moles of acid, I can work out its concentration. Rearranging the equation, concentration = moles / volume. Again, remember to convert volume in dm3.
25 cm3 = 0.025 dm3
Concentration = 8.15 x 10-3 / 0.025 = 0.326 mol dm-3
And that’s all there is to it! The steps we carried out have been summarised below:
- Make sure you have a balanced symbol equation so you can identify the molar ratios between your acid and base.
- Calculate the moles of the thing of KNOWN CONCENTRATION by multiplying the concentration with the titre volume. Remember to convert cm3 to dm3.
- Use the acid:base molar ratios to work out the moles of the thing of UNKNOWN CONCENTRATION.
- Calculate the concentration of the thing of UNKNOWN CONCENTRATION by dividing the moles by the volume. Again, remember to convert volume in cm3 to dm3.