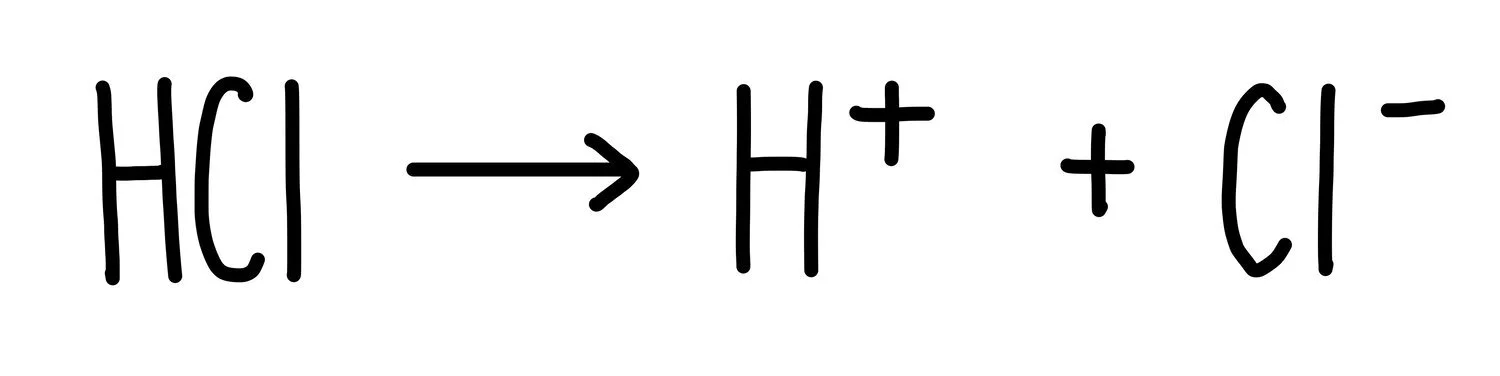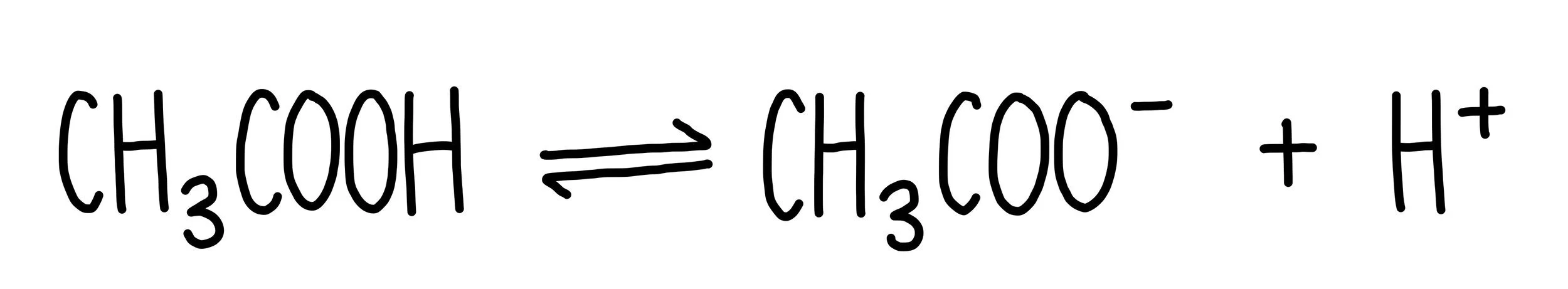Acids, Alkalis and Titrations
Acids are compounds which release hydrogen ions, and alkalis are compounds which release hydroxide ions. Acids can neutralise alkalis to form a neutral solution. Titrations are used to determine the concentration of an acid or alkali..
Indicators
We can use indicators to distinguish between acidic and alkaline solutions. Indicators are dyes which change colour depending on the pH of the solution. The most commonly used indicators are described below - make sure you remember the colour changes for each.
Litmus: litmus paper is red in acidic solutions, purple in neutral solutions and blue in alkaline solutions. To see the colour change, you need to use blue paper to test acidic solutions and red paper to test alkaline solutions.
Phenolphthalein: colourless in acidic solutions and bright pink in alkaline solutions.
Methyl orange: red in acidic solutions and yellow in alkaline solutions.
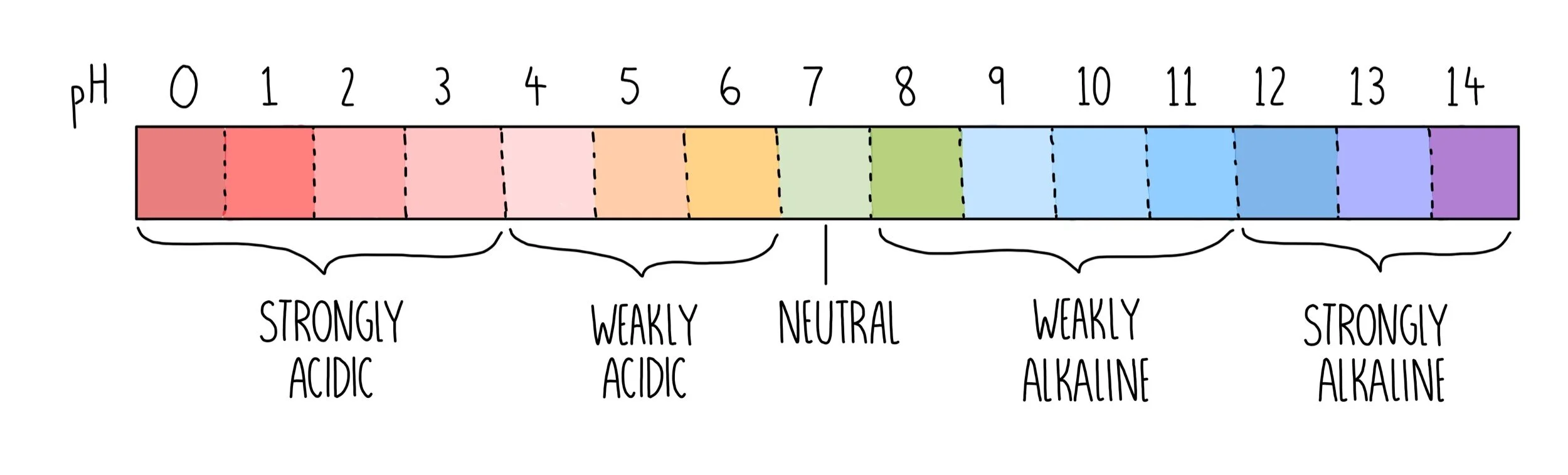
pH scale
The pH scale goes from 0 to 14. A very strong acid will have a pH of 0-1 whereas a very strong alkali will have a pH of 13-14. Pure water which is a neutral substance will have a pH of 7.
Universal indicator can be used as a more precise measurement of pH. It gives a range of different colours depending on the pH of a solution (shown above) which can be compared to a colour chart to give a more accurate pH value.
Acids and Alkalis
Acids are a source of hydrogen ions (H+) and alkalis are a source of hydroxide ions (OH-). A base is something which will become an alkali when added to water. For example, magnesium oxide is a base because it will dissolve in water to form magnesium hydroxide (Mg(OH)2) which is an alkali because it is a source of hydroxide ions.
Alkalis can neutralise acids (or vice versa) - hydrogen ions combine with hydroxide ions to form water, forming a neutral solution. The equation for the reaction is:
Titrations
Titrations are used to find out the concentration of an acid or an alkali. Let’s say we have an acid of an unknown concentration, we can react it with an alkali of known concentration and record the volume needed for neutralisation to calculate its concentration. The method for a titration is described below:
Using a pipette, add a measured volume of alkali into a conical flask along with a few drops of indicator.
Fill a burette with acid and record the starting volume.
Turn the tap on the burette to slowly add acid to the alkali until the indicator changes colour (neutralisation has occurred at this point).
Record the volume of acid added to the alkali.
Repeat the titration until you get concordant titres - results that are very similar to each other so that you know you have reliable data.
Titrations also work the other way round, where alkali is added to the acid.
Worked example: titration calculation
A student titrated 60 cm3 of hydrochloric acid to 1 mol dm-3 solution of sodium hydroxide, which had a volume of 30 cm3. Calculate the concentration of the hydrochloric acid.
- The first thing we need to do is calculate the moles of the substance with a known concentration. In this case, it's the sodium hydroxide. To calculate moles, we need to convert cm3 to dm3 by dividing by 1000. Then we can multiply concentration and volume to find out the moles.
- 30/1000 = 0.03 dm3 x 1 mol dm-3 = 0.03 mol
- The next thing is to write an equation for the reaction. Here, the equation is: HCl + NaOH --> NaCl + H2O
- From the equation we can see that there is a 1:1 ratio between the acid and alkali, so if we have 0.03 mol of NaOH then we also have 0.03 mol of HCl.
- Now that we know the moles and volume of HCl, we can work out its concentration. 0.03 / (60/1000) = 0.5 mol dm3.
Strong and weak acids
Strong acids, like hydrochloric acid, completely ionise in aqueous solutions. This means that every single HCl molecule breaks apart into hydrogen ions and chloride ions. This is what gives it such a low pH, since it releases hydrogen ions more readily than weaker acids. Nitric acid and sulfuric acid are also examples of strong acids.
A weak acid, such as ethanoic acid, only partially ionises in aqueous solutions. This means that some ethanoic acid molecules will release a hydrogen ion but others will remain completely intact. We represent partial ionisation of weak acids using a double-headed arrow. Other weak acids include citric acid and carbonic acid. You find these acids in various food items – ethanoic acid is an ingredient in vinegar, citric acid is found in citrus fruits and carbonic acid is found in fizzy drinks. The fact that these acids only partially ionise means that they are safe to consume. Start gulping hydrochloric acid and you’re in trouble.
The stronger the acid (i.e. the more readily it releases hydrogen ions), the lower the pH. This is why hydrochloric, sulfuric and nitric acids have a very low pH (around 1-2) whereas ethanoic and citric acids have a higher pH (around 3-6). As the pH decreases by 1 unit, the hydrogen ion concentration of the solution increases by a factor of 10. For example, a solution with a pH of 4 has 10x as many hydrogen ions compared to a solution with a pH of 5. A pH difference of 2 means that there is 100x difference in hydrogen ion concentration and a pH difference of 3 means a 1000x difference in hydrogen ion concentration, and so on.