Analytical Techniques
Infrared spectroscopy
Infrared spectroscopy (IR) is used to identify a molecule’s functional groups. It works by beaming infrared radiation through a sample. The IR radiation is absorbed by the covalent bonds, causing them to vibrate.
Since different bonds vibrate at different frequencies, IR allows us to identify the types of bonds that are present. In the table below, you can see the range of frequencies at which different bonds vibrate when they absorb IR radiation.
The IR spectrometer will produce a spectrum. To identify the functional groups that are present, we just need to read off the wavenumber of the major peaks. Don’t worry about memorising these – they will be given to you on the data sheet.
In exam questions where they ask you to identify a compound from a given IR spectrum, it may be necessary to point out what isn’t there. For example, the presence of a peak at around 3000 cm-1 (-OH) along with the absence of a peak at around 1700 cm-1 (C=O) indicates that the compound is an alcohol, not a carboxylic acid.
Infrared spectroscopy is used in breathalysers in roadside drink driving tests — it works by detecting the C-H bond from ethanol.
IR is also used to monitor the concentrations of polluting gases in the atmosphere. For example, to measure how much nitrogen monoxide is present in an air sample, the IR spectrometer will determine the abundance of the N=O bond.
Mass spectrometry
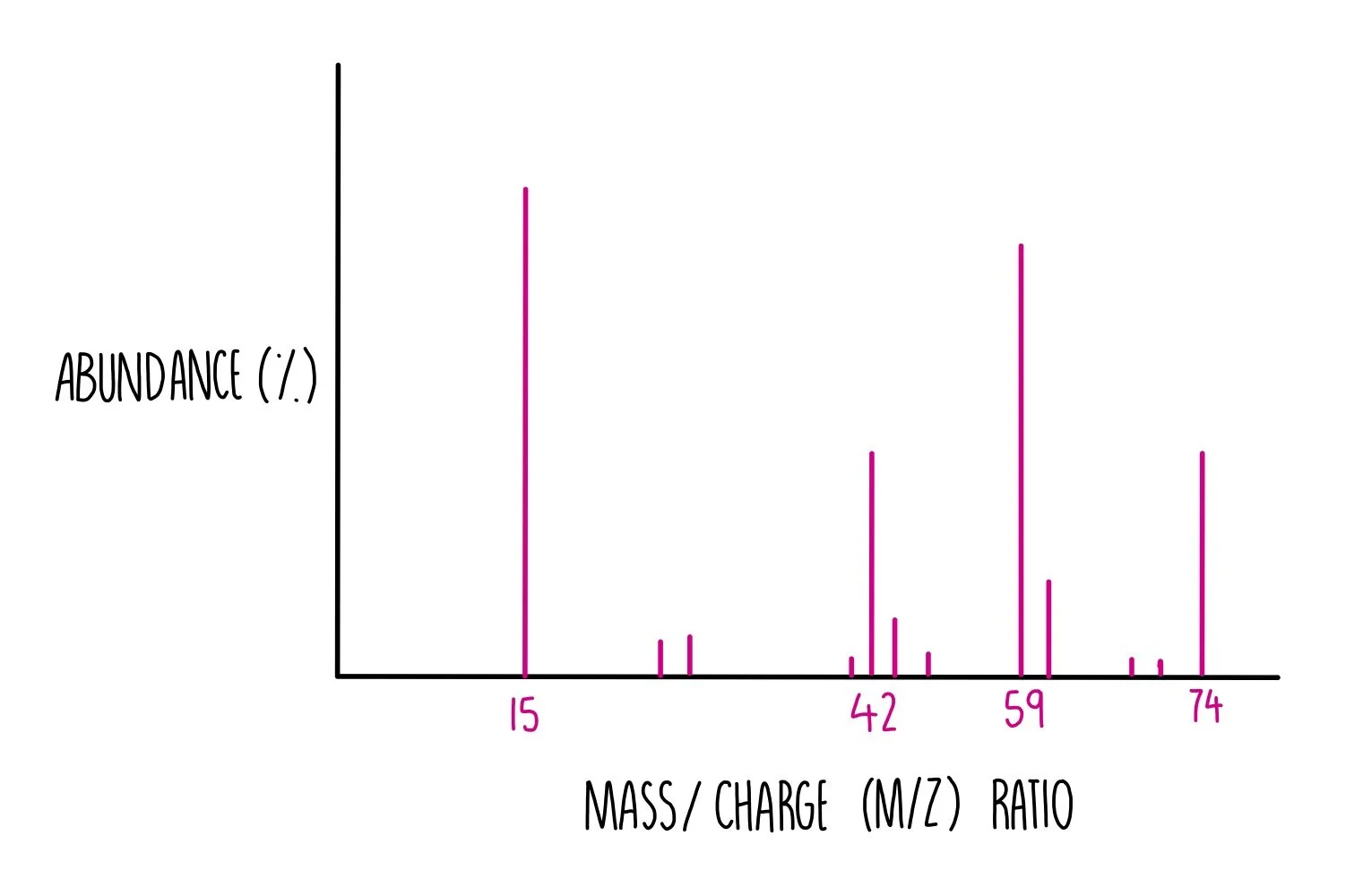
Mass spectrometry can also be used to identify unknown compounds. It tells us the Mr of the compound and the mass of fragments that the compound may have split into.
It works by bombarding electrons at the sample, knocking off an electron and creating a +1 ion. The mass spectrometer produces a mass spectrum, which has mass/charge (m/z) along the x-axis and the percentage abundance of the fragment on the y-axis. Since +1 ions are formed, the m/z value will be equal to the mass of that fragment. The very last peak, known as the M peak, tells us the Mr of the whole compound.
Have a look at the spectrum below to see how you can determine the structure of an unknown alcohol.
- The M peak is at 74, so we know that the Mr of the whole compound is 74.
- There are molecular ion peaks at 15, 42 and 59. Using trial-and-error, add the Mr’s of carbon, hydrogen and oxygen until you reach these numbers.
- The peak at m/z 15 is likely to be CH3+
- The peak at m/z 42 is likely to be CH3CCH3+
- The peak at m/z 59 is likely to be CH3COH(CH3)+
- The compound must be 2-methylpropan-2-ol.
Combined techniques
It’s very common to get an exam question where you’re expected to use both of these techniques together along with its mass or percentage composition. Have a look at the worked example below to see how this is done.
Worked example – using mass spectrometry and IR spectroscopy to identify an unknown compound
Compound G is a branched-chain organic compound that does not have E and Z isomers. Elemental analysis of compound G gave the following percentage composition by mass: C, 55.8%; H, 7.0%; O, 37.2%. Using the percentage composition, mass spectrum and infrared spectrum, determine the structure of compound G.
Let’s start by annotating the infrared spectrum to see what functional groups are present.
We can use the percentage composition to work out the compound’s empirical formula.
Now let’s look at the mass spectrum to see what the molecular formula must be. We can also work out the identity of fragments from their masses.
Now we can put all that information together to come up with a compound that fits. You might need to do a bit of trial and error. Because of the lack of hydrogens, we know it must be an alkane. From the infrared spectrum, we know it contains a carboxylic acid functional group. The information provided in the question also explained that we’re dealing with a branched molecule.
Therefore the structure must be: