Atomic Structure
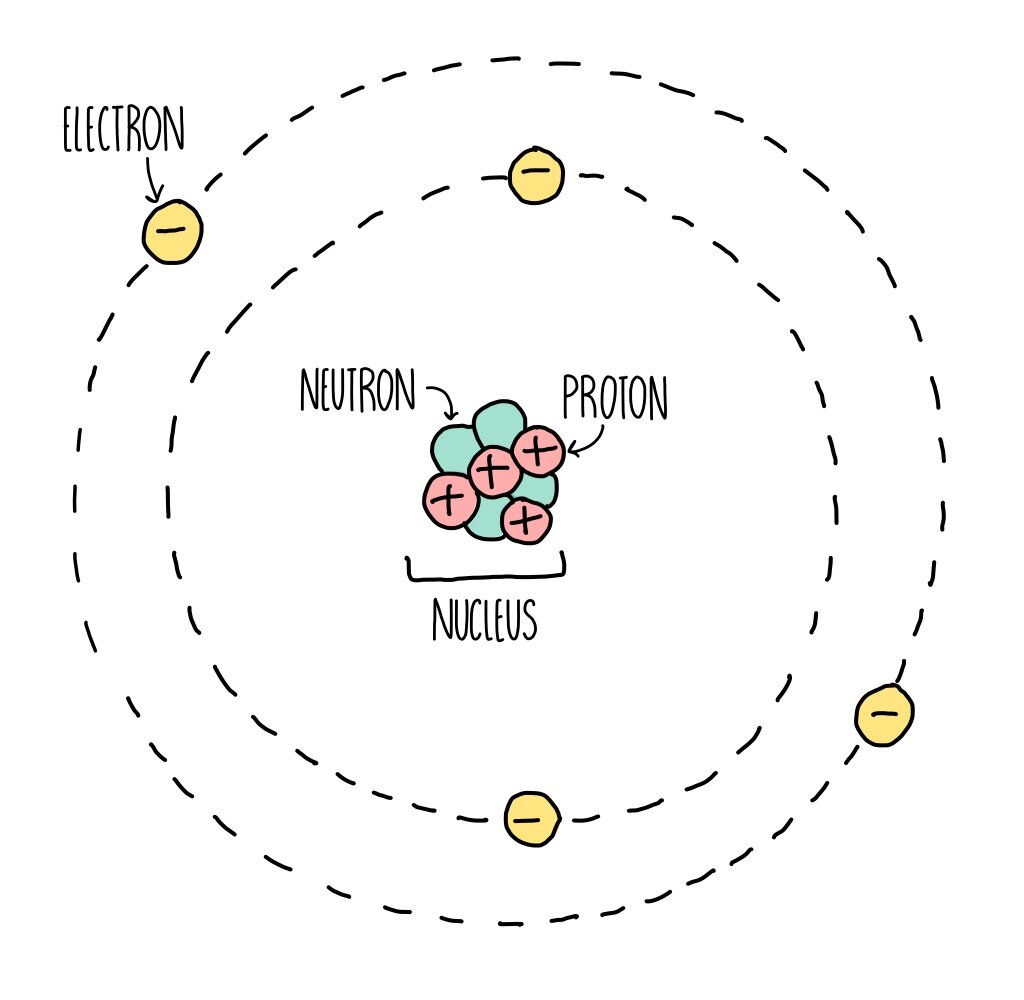
Everything around us is made up of atoms - the ‘basic building blocks’ of matter. An atom is made up of three different types of particle, protons, neutrons and electrons.
Atomic structure
Every atom is made up of a central nucleus surrounded by electrons whizzing around the nucleus in electron orbitals. The nucleus is tiny in relation to the size of the whole atom - comparable to a pea in the middle of a football stadium.
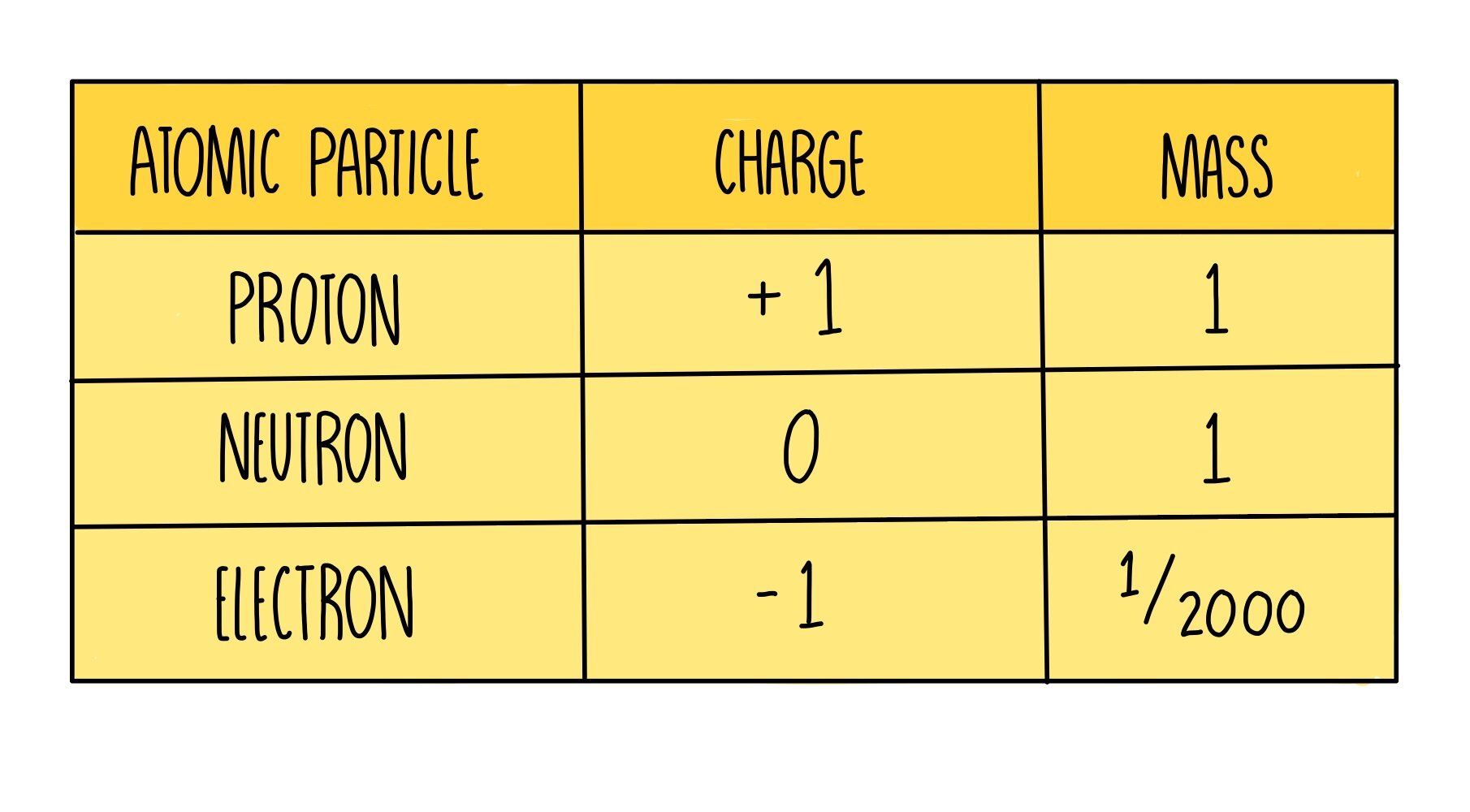
The nucleus is made up of protons and neutrons, which are the heaviest particles. Electrons are approximately 2000 times lighter than a proton or neutron and weigh practically nothing, therefore most of the mass comes from the tiny nucleus.
Protons are positively charged whereas neutrons have no charge, making the nucleus positively charged overall. Electrons are negatively charged, which enables the nucleus to keep hold of its electrons, due to the attraction between the opposite charges.
Atomic number & mass number
Each atom has an atomic number and a mass number, which are displayed on the Periodic table as the small number and larger number next to each chemical symbol.
The atomic number tells us the number of protons which is unique for each element. If the number of protons changes (and therefore the atomic number changes), which could occur during radioactive decay, for example, then the identity of the element also changes.
The mass number tells us the number of both protons and neutrons in the nucleus. Remember that most of an atom’s mass comes from the nucleus and that the nucleus is made up of both protons and neutrons. Two atoms of the same element can have different mass numbers because they are isotopes.
Isotopes
Isotopes are atoms which have the same number of protons but different numbers of neutrons. Therefore they have the same atomic number but a different mass number. For example, the most common isotopes of carbon are carbon-12 and carbon-13, in which carbon-13 has an additional neutron in its nucleus to give it a higher mass number.
The mass numbers that we see in the periodic table are an average of all the masses of all isotopes of a particular element. We call this the relative atomic mass (RAM) and can be calculated using the following formula:
Lets say we have a sample of carbon which consists of 76% of the isotope carbon-12 and 24% carbon-13, then the relative atomic mass (RAM) would be:
RAM = (76 x 12) + (24 x 13) / 100 = 12.24
Models of the atom
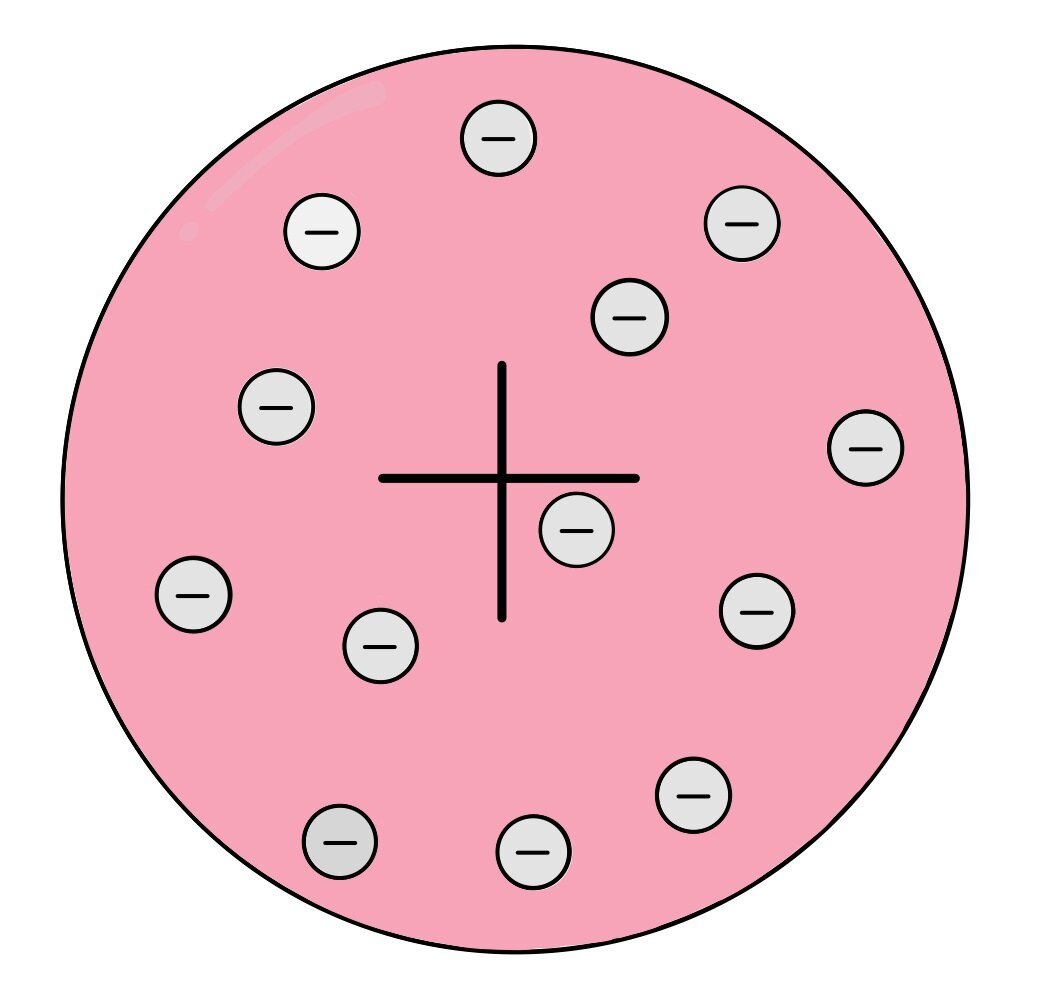
Science is not static – we’re constantly updating scientific models as new research and evidence becomes available. This is the case for the model of atomic structure. Before the electron was discovered, atoms were thought to be the smallest things that existed. When the electron was identified, scientists realised that the atoms could be divided into even smaller subatomic particles, leading to the plum pudding model of the atom. This model suggested that the atom exists as a ball of positive charge with negative electrons scattered in it, like raisins in a plum pudding.
In 1905, Ernest Rutherford carried out an experiment which disproved the plum pudding model. His experiment involved shooting a beam of alpha particles at a thin piece of gold foil suspended in a vacuum. If the plum pudding model was correct, the alpha particles should all be repelled from the atoms in the gold foil, since the atom was thought to be positively charged and alpha particles are also positively charged (remember that like charges repel each other). Instead, Rutherford found that most of the alpha particles passed straight through the gold foil, with only a very small number being repelled. This led him to conclude two things:
The atom is mostly empty space, with a tiny nucleus located in its centre
The nucleus of the atom is positively charged
As a result of Rutherford’s findings, the nuclear model of the atom was proposed which replaced the plum pudding model. The nuclear model suggests that the atom is made up of a small central nucleus with electrons whizzing around it.
The nuclear model was updated when Niels Bohr discovered that electrons don’t move around just anywhere outside the nucleus but occupy specific energy levels (orbitals). The new atomic model was similar to the nuclear model, except that the electrons moved in orbitals at fixed distances around the nucleus.
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles. The experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus. This was about 20 years after the nucleus became an accepted scientific idea.
Electronic Structure
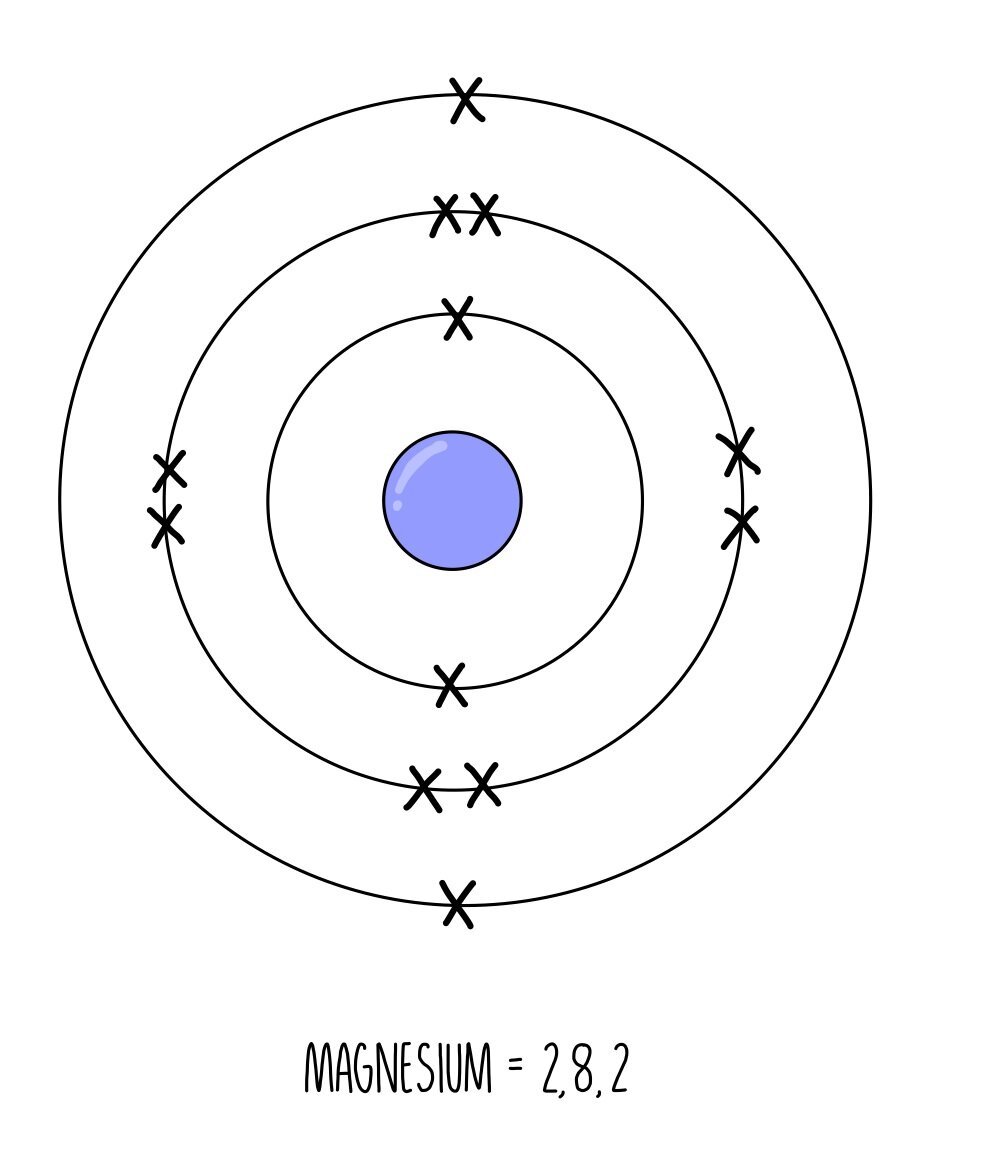
An atom will have the same number of electrons as the number of protons in its nucleus, which means the amount of electrons will be the same as its atomic number. Since each successive element in the periodic table has one more proton than the last, they also have an additional electron. Electrons occupy specific energy levels around the nucleus which can only hold a fixed number of electrons. The innermost energy level holds a maximum of two electrons then the following energy levels can hold eight electrons each. Just like collecting Pokemon cards as a kid, atoms are always eager to get a complete set and will share, steal or throw away electrons until their electron shells are completely occupied with electrons.
To represent electron configuration we write the number of electrons in each energy level, separated by commas. For example:
Magnesium has 12 electrons with an electron configuration of 2, 8, 2.
Chlorine has 17 electrons with an electron configuration of 2, 8, 7.
Notice that the last number is always the same as the group number that element is found in. Magnesium is in group 2 and has two outer electrons whereas chlorine is in group 7 so has seven outer electrons. The number of electrons in the outer shell determines how an element reacts. Therefore, all elements found in the same group have similar chemical properties because they have the same number of outer electrons.
The last group on the Periodic Table are the Noble gases. These have a complete outer energy level with eight electrons in their outer shell. A full energy level makes these elements very stable, with no need to take or give away electrons to other elements, therefore these elements are very unreactive.