Atoms, Compounds, Molecules and Equations
Introduction
Atomic Structure and Isotopes
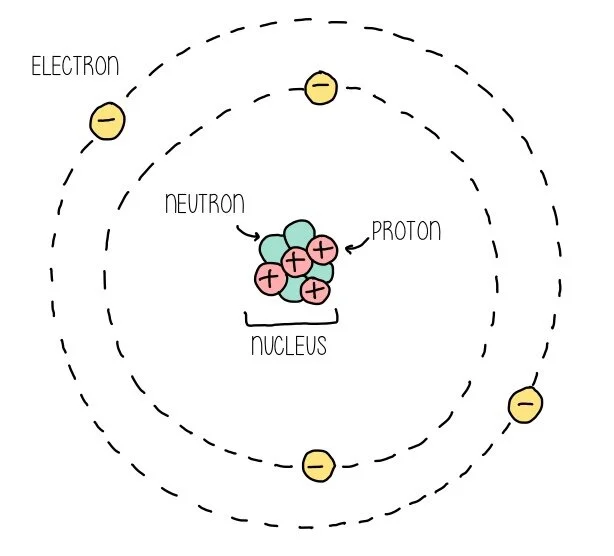
You should remember from GCSE that all atoms are made up of three different types of sub-atomic particle. You’ve got the protons and neutrons in the nucleus of the atom and electrons whizzing around the nucleus in discrete energy levels. The atomic number is the number of protons in the nucleus of an atom and confers the identity of the element. This means that if the number of protons in the nucleus changes due to radioactive day, the identity of the element itself also changes (beta decay could convert uranium into thorium, for example). The mass number is the number of protons and neutrons in the nucleus. When we talk about mass numbers of an element, we are describing the mass of an element in comparison to carbon. The relative atomic mass is therefore defined as the average mass of one atom of an element compared to 1/12th of the mass of an atom of carbon-12. There’s no special reason carbon is used as a comparison - a while ago oxygen-16 was used as the standard for atomic weights.
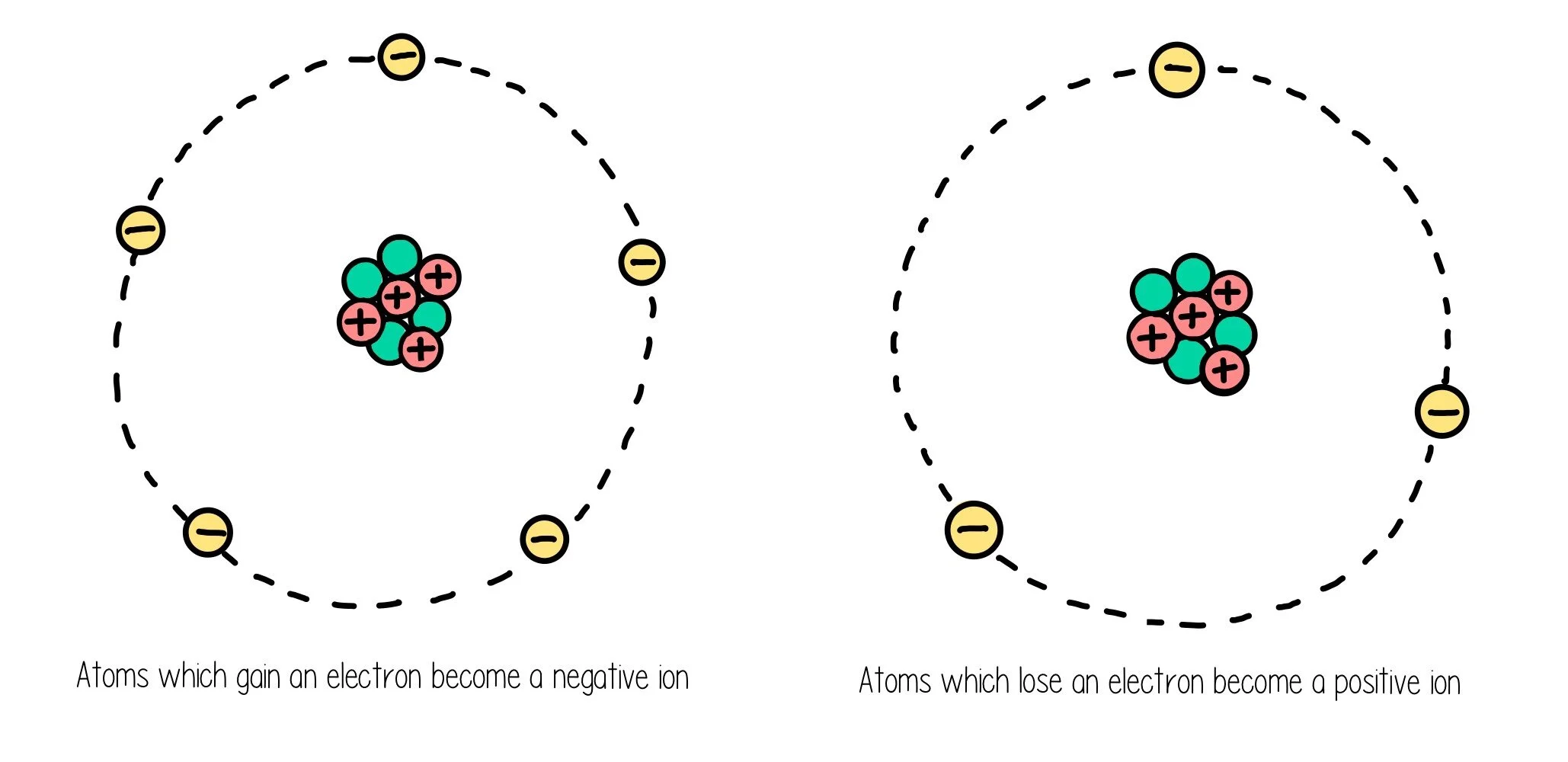
Atoms have an overall neutral charge because they have an equal number of positive protons and negative electrons, which means the atomic number is equal to the number of electrons for a neutral atom. When atoms gain electrons, they will become a positive ion and when they lose electrons they become a negative ion.
Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This means that isotopes have the same atomic number but will have different mass numbers. If we compare the isotopes carbon-12 and carbon-13, both will have six protons in their nucleus but carbon-13 will have an additional neutron compared to carbon-12 (7 neutrons compared to 6).
Relative mass
Since elements can exist in different forms with various numbers of neutrons, we describe an element in terms of its relative atomic mass, which is the average of all the different isotopes of an element in the proportions in which they are found. Let’s say we have a sample of copper, of which 70% consists of the isotope copper-63 and the remaining 30% is the isotope copper-65. The relative atomic mass of the sample will an average of the two masses and can be calculated using the following equation:
Relative atomic mass = (mass of isotope 1 x percentage) + (mass of isotope 2 x percentage) / 100
Therefore to work out the relative mass of copper in our sample: (63 x 70) + (65 x 30) / 100 = 63.6
Mass spectrometry is used to determine the masses and relative abundance of different isotopes. A vapourised sample is fed into the spectrometer and the sample is ionised using an electron gun. When electrons are fired at the sample, this knocks an electrons off the atoms in the sample, forming +1 ions. The atoms then pass through the mass spectrometer, with the heavier atoms moving much slower than their lighter counterparts. At the end of the spectrometer, they reach the detection plate which is loaded with electrons. The positive ions gain an electron and are converted back into their neutral form. This movement of electrons generates an electric current which is proportional to the abundance of that particular isotope.
Formulae of ions
You need to be able to describe the charge of ions formed from certain atoms or compounds. Some are easy to predict based on their location on the Periodic Table whereas you may need to memorise others.
Based on their positions in the Periodic Table and our knowledge of the octet rule, we know that:
Group 1 elements will form 1+ ions after they lose their single outer electron
Group 2 elements will form 2+ ions after losing their two outer electrons
Group 3 elements will form 3+ ions after losing their three outer electrons
Group 5 elements will form 3- ions after gaining three electrons to complete their octet
Group 6 elements will form 2- ions after gaining two electrons to complete their octet
Group 7 elements will form 1- ions after gaining one electron to complete their octet
If you don’t know the charge of the ions listed below, you’ll have to memorise them
| Ammonium | NH4+ |
| Silver | Ag+ |
| Zinc | Zn2+ |
| Hydroxide | OH- |
| Nitrate | NO3- |
| Carbonate | CO32- |
| Sulfate | SO42- |