Carbohydrates and Lipids
Carbohydrates
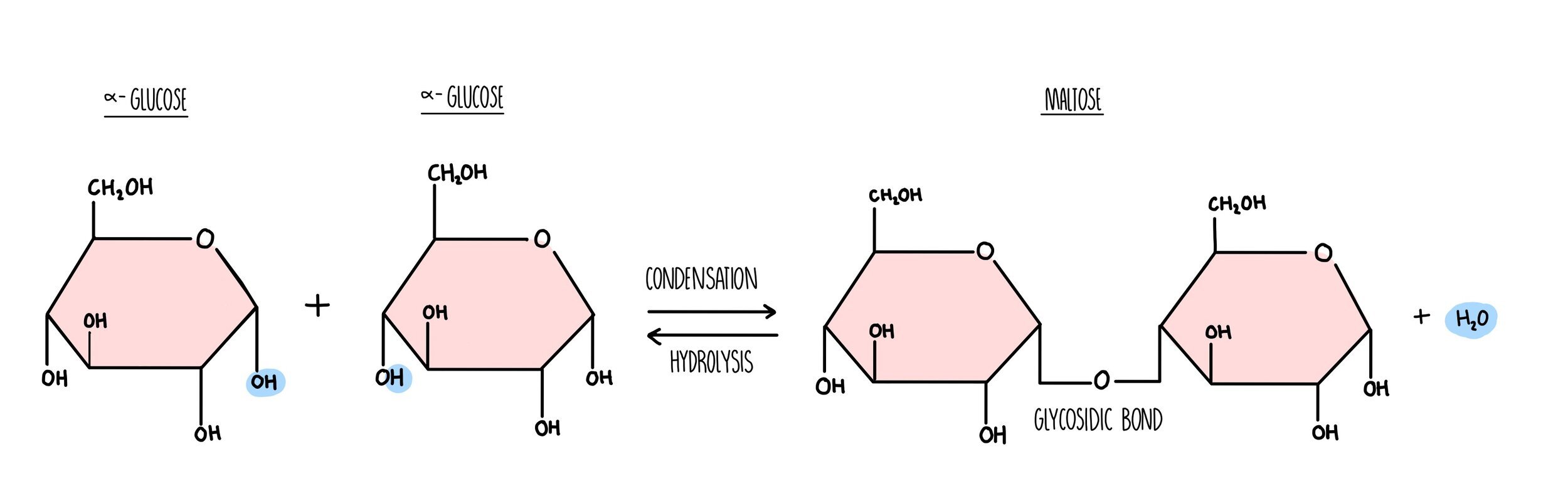
Carbohydrates are long chains of sugar molecules (the technical term is ‘polysaccharides’) formed from lots of individual sugar molecules (monosaccharides) joining together. When two monosaccharides react to form a disaccharide, a condensation reaction occurs. During the condensation reaction, a molecule of water is removed (from a hydroxyl group on one sugar and a hydrogen on another) and a glycosidic bond forms. When polysaccharides are broken down during digestion, a hydrolysis reaction occurs in which a water molecule is added to break the glycosidic bond.
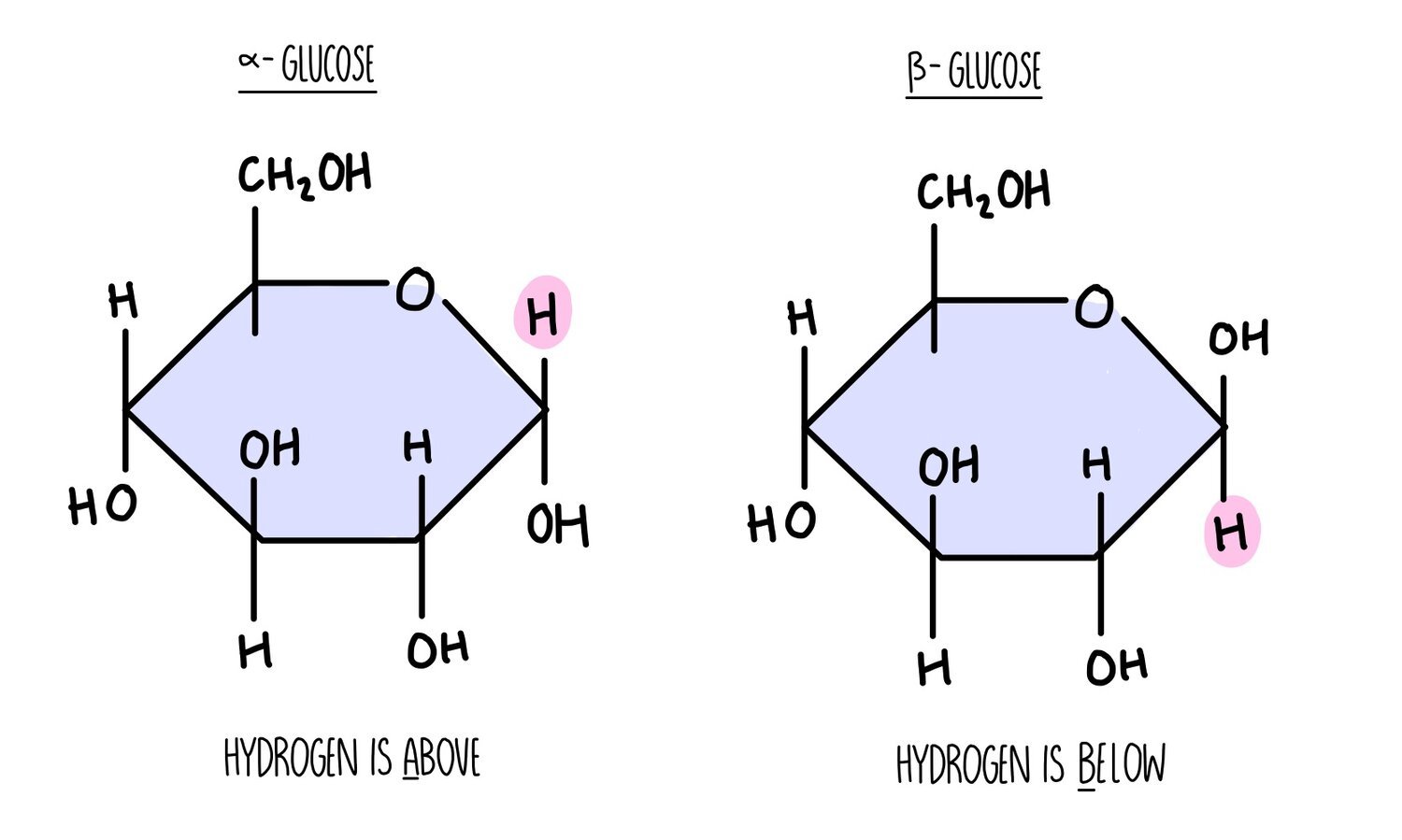
Carbohydrates are made up of carbon, hydrogen and oxygen only. Monosaccharides such as glucose and fructose consist of a ring structure with the general formula CH2O. Glucose is a hexose sugar, which means it is made up of six carbon atoms. It can exist as two different forms, alpha and beta which differ in the position of the hydrogen and hydroxyl groups on the right-hand carbon. An easy way to tell the difference between them is:
Alpha - the hydrogen atom is above the carbon.
Beta - the hydrogen atom is below the carbon.
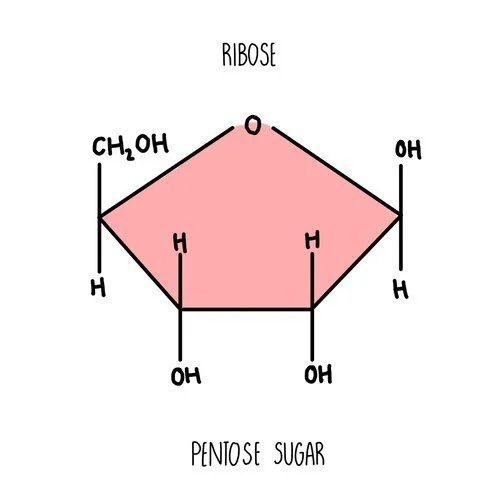
Ribose is another example of a monosaccharide - it’s a component of RNA molecules. It is a pentose sugar with five carbon atoms in the ring.
Polysaccharides
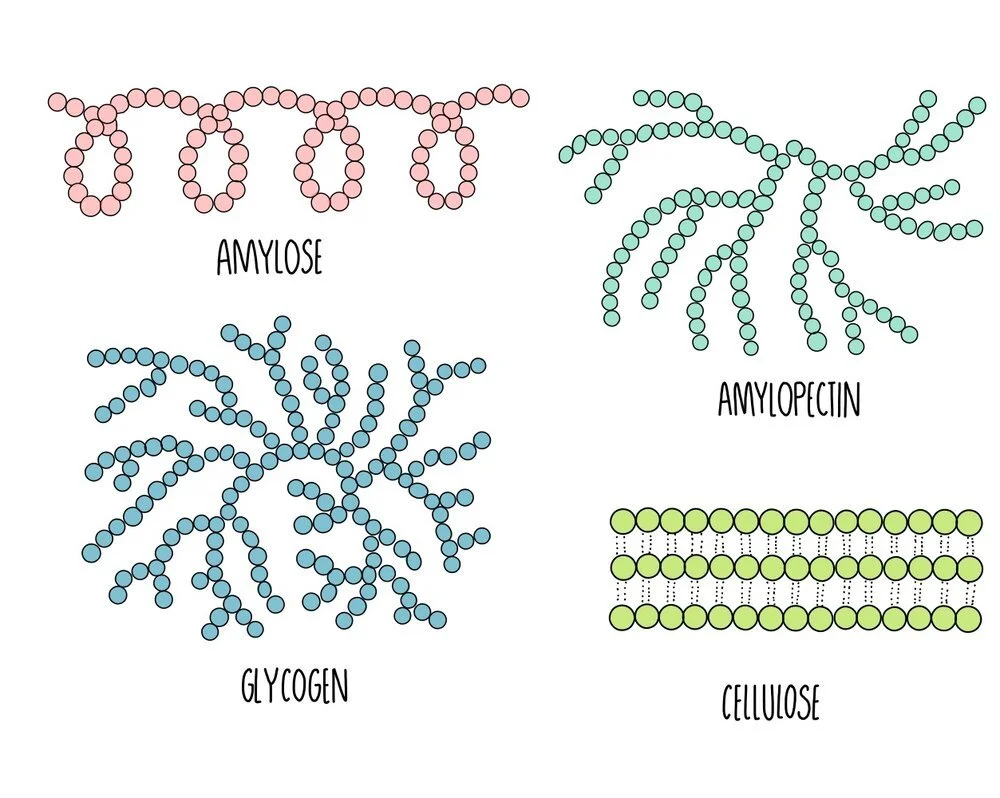
The polysaccharides found in plants are starch and cellulose. Starch is broken down by the plant when it needs energy and cellulose is the major component of plant cell walls. Starch exists in two different forms: amylose and amylopectin. In animals, the energy storage carbohydrate is glycogen.
Amylose: unbranched spiralling chains of alpha-glucose molecules. Its coiled structure means that it is very compact so lots of amylose can be packed into a cell.
Amylopectin: branched chains of alpha-glucose molecules. Its branches increase its surface area which means that enzyme can quickly break it apart when glucose is needed for respiration.
Cellulose: long unbranched chains of beta-glucose molecules. Multiple chains are linked together by hydrogen bonding to form strong structures called micofibrils. The strong microfibrils in the cell wall help to give plant cells their shape and structural support.
Glycogen: branched chains of alpha glucose, similar to amylopectin but with more side-branches. This gives it a large surface area for enzyme action to release glucose when energy is needed. It is more compact that amylopectin, which means more can be stored in a cell.
Lipids
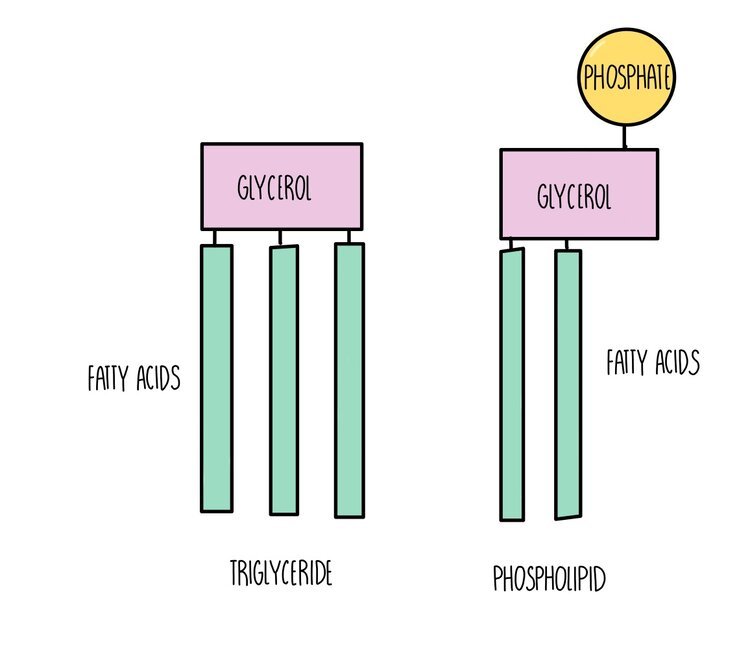
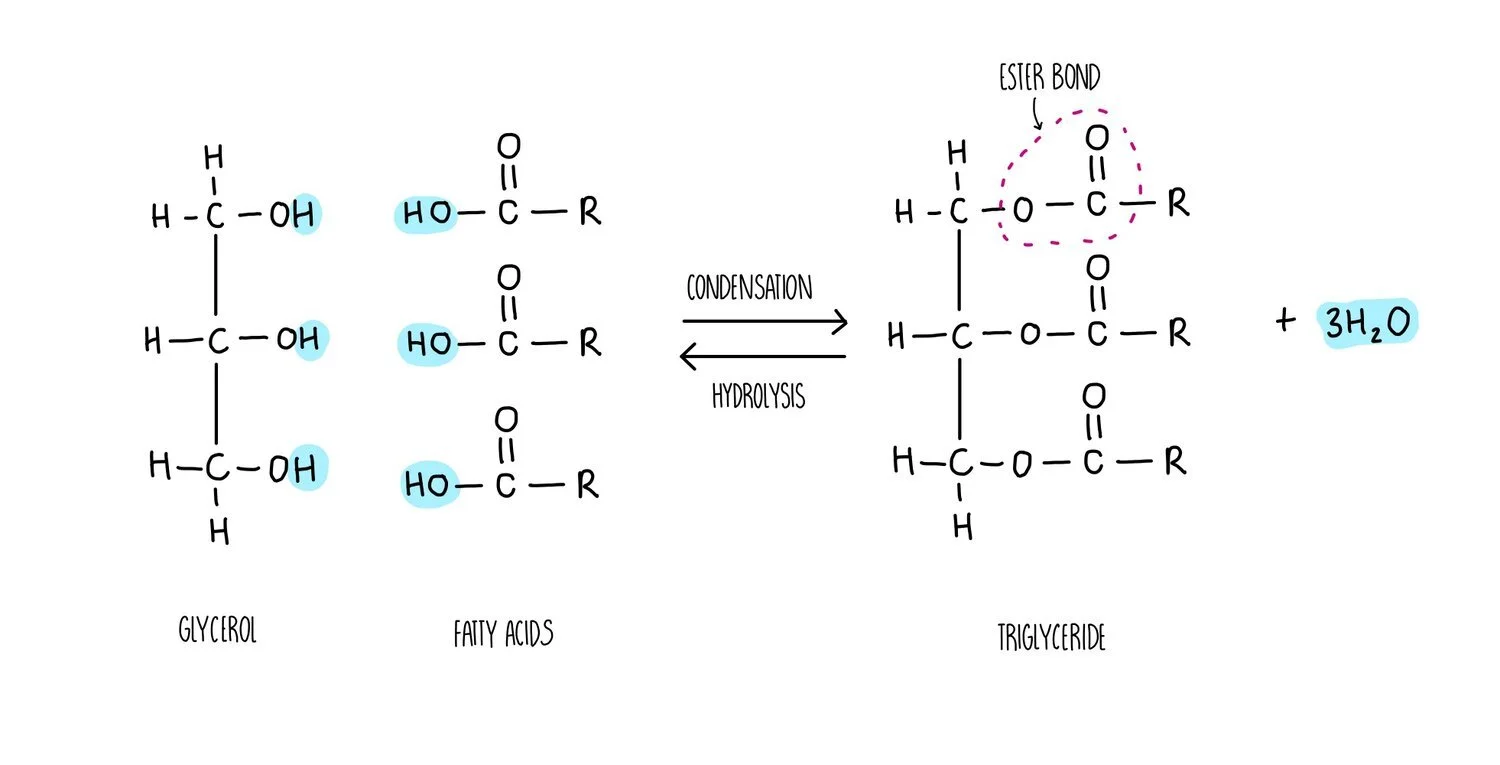
Triglycerides, phospholipids and cholesterol are all types of lipid. Triglycerides and phospholipids have similar structures as they are both made up of glycerol connected to fatty acids molecules through ester bonds. However, triglycerides contains three fatty acid ‘tails’ whereas phospholipids only have two. Phospholipids also possess a phosphate group which is absent in triglycerides. The fatty acid tails of triglycerides or phospholipids can be saturated (only single carbon-carbon bonds) or unsaturated (contain at least one double carbon bond).
The synthesis of a phospholipid or triglyceride from glycerol and fatty acids involves the formation of an ester bond. Just like we saw for the formation of a glycosidic bond, the synthesis of ester bonds is a condensation reaction in which a water molecule is released. Breaking apart a triglyceride or phospholipid involves the addition of water in a hydrolysis reaction.
Different types of lipids have different functions in living organisms:
Triglycerides: used as an energy store. A lot of energy is released when the ester bonds are hydrolysed - around twice as much compared to the breakdown of carbohydrates. Inside cells, triglycerides group together into lipid droplets where the hydrophobic tails face inwards and the hydrophilic heads face outwards. These insoluble droplets make good energy storage molecules since they do not affect the osmotic potential of the cell.
Phospholipids are the main component of cell membranes. They form a phospholipid bilayer with the hydrophobic tails facing each other and the hydrophilic heads facing outwards, forming a barrier to prevent any polar molecules from entering or leaving the cell.
Cholesterol is also found in cell membranes and helps to strengthen the membrane. Cholesterol pushes the hydrophobic tails of phospholipids closer together, making the membrane more rigid.
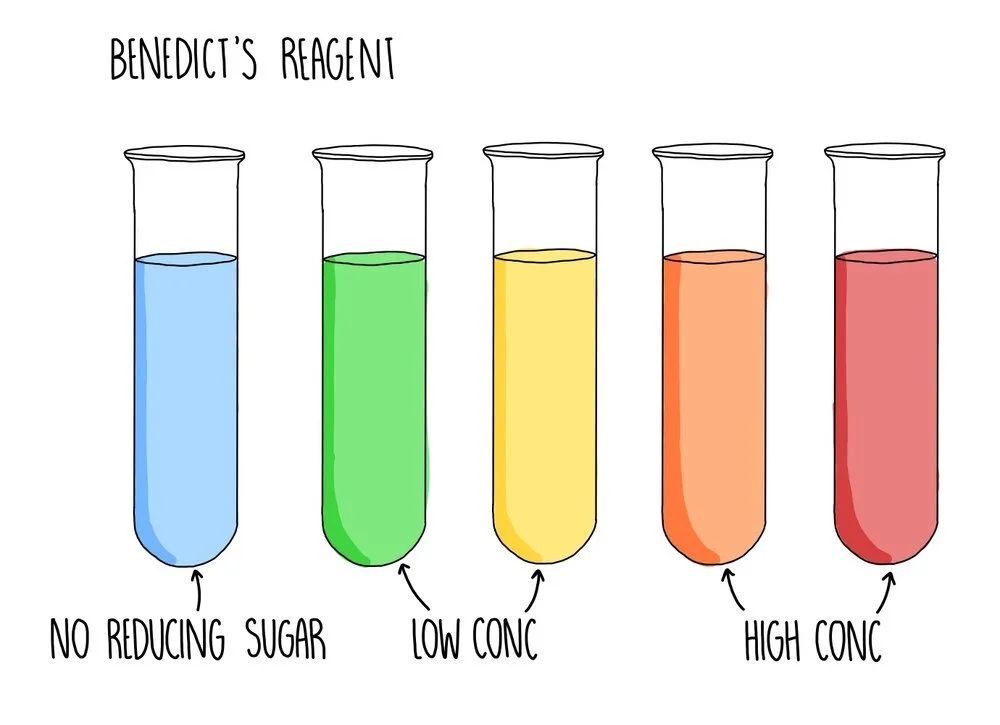
Testing reducing sugars using Benedict’s solution
A reducing sugar is any sugar that can act as a reducing agent because of its aldehyde or ketone group. All monosaccharides are reducing sugars, along with some disaccharides (such as maltose and lactose). If the sugar you are testing is a reducing sugar, it will change colour from blue to green/yellow/orange/brick red, depending on the concentration of sugar you are testing. Low concentrations of reducing sugar will cause a green or yellow precipitate to form whereas a reducing sugar of higher concentration will appear orange or brick red.
If the test for reducing sugars is negative and you want to confirm the presence of a non-reducing sugar, such as sucrose, you need to break down the sample into monosaccharides using hydrochloric acid which breaks the glycosidic bonds. The sample is neutralised with sodium hydrogencarbonate and the Benedict’s test can be carried out. Since sucrose will have been broken down into glucose and fructose (both reducing sugars), you should now see a positive result if your original sample contained sucrose.
If you want to accurately measure the amount of reducing sugar in a sample, you can use calorimetry. Calorimetry works by measuring how much light is able to pass through the solution. The less light that passes through, the higher the absorbance of the sample and the higher the concentration. Before measuring the unknown sample, you first need to measure the absorbance readings of a series of solutions of a known concentration. You would plot a calibration curve of absorbance against concentration then measure the absorbance of the sample of unknown concentration. You can read off your graph to find the concentration which corresponds to this particular absorbance reading.
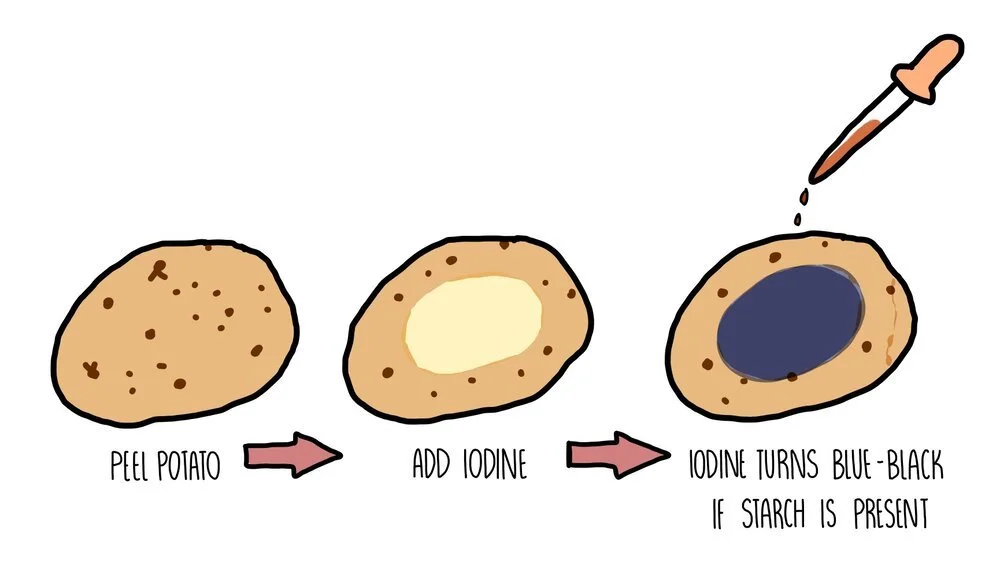
Testing for starch using iodine
Starch is the storage carbohydrate in plant cells. If you want to test a part of a plant (such as a potato) for starch, you need to add iodine dissolved in potassium iodide solution. A positive result occurs when the solution changes colour from orange/brown to blue/black.
Testing for lipids using the Emulsion test
To test for the presence of lipid in a sample, you need to add ethanol to a sample and mix thoroughly by shaking. Add an equal volume of water and if lipid is present a milky white suspension should form. If the solution remains colourless, no lipid was present in the sample.