Cell Membranes
Fluid mosaic model
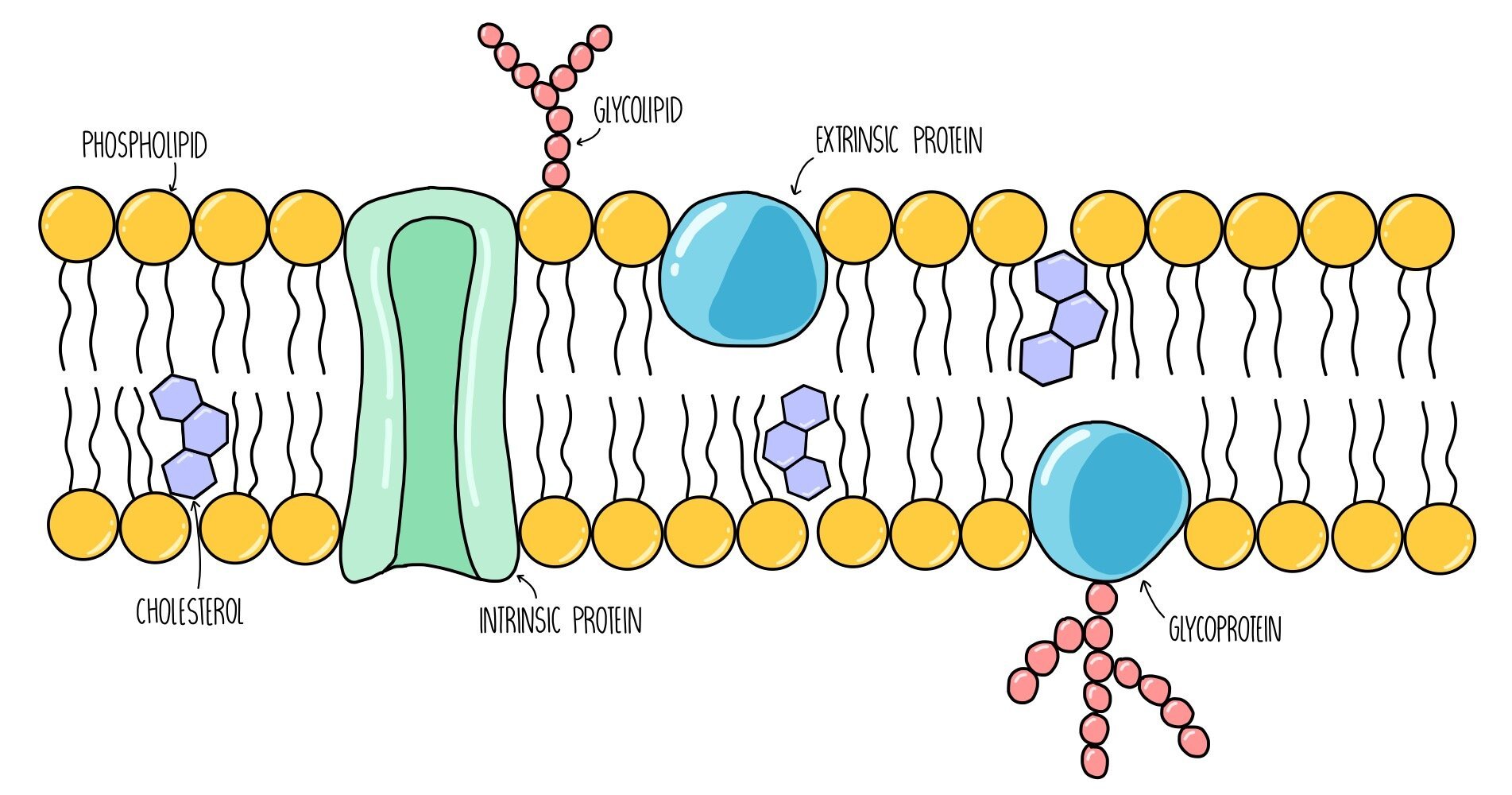
The structure of the plasma membrane is made up of a bilayer of phospholipids with proteins and cholesterol interspersed throughout the structure. The fluid mosaic model is used to describe the arrangement of molecules in the membrane - ‘fluid’ because the phospholipids are constantly moving around and ‘mosaic’ because protein molecules are scattered throughout the phospholipids like tiles in a mosaic.
We refer to this concept as a ‘model’ because it is the best representation of membrane structure based on the evidence which is currently available. As we learn more about the structure of the plasma membrane, the fluid mosaic model may be updated.
Components of the plasma membrane
Phospholipids: consist of a hydrophilic head group which faces the intracellular / extracellular fluid and two hydrophobic tails which point towards each other, away from water. They are the main component of the plasma membrane and form a barrier to anything which is not lipid-soluble (such as ions and glucose).
Glycoproteins: these are proteins with sugar molecules attached. They act as recognition sites and antigens - antigens are like little ‘flags’ on the surface of our cells which allows our body to detect which cells are our own and which cells are foreign.
Glycolipids: these are phospholipids with sugar molecules attached. They have a similar function to glycoproteins - they also act as recognition sites and antigens. They also increase membrane stability by forming hydrogen bonds with water molecules.
Cholesterol: cholesterol is a lipid which slots in between the phospholipid tails, pushing them closer together. It regulates the stability and fluidity of the plasma membrane.
Intrinsic proteins: these are proteins which span both bilayers of the plasma membrane. They act as channels or carrier proteins to transport water-soluble molecules.
Extrinsic proteins: these are proteins which are found on the surface of the plasma membrane. They usually function as enzymes and catalyse chemical reactions inside the cell.
Investigating cell membrane structure
The permeability of cell membranes is affected by things like temperature, pH and ethanol. You may be asked to describe an experiment to determine the effect of one of these factors on membrane permeability. These experiments using involve plant cells which contain a coloured pigment, such as beetroot, since we can measure the amount of membrane permeability depending on how much pigment leaks out of the cells and into the surrounding solution. The method for this type of experiment is outlined below:
- Prepare eight cylinders of beetroot of equal size. Make these samples as similar as possible, e.g. by cutting from the same part of each plant. Rinse each piece to remove any pigment released during cutting.
- If you are investigating the effect of temperature, prepare eight water baths of varying temperatures ranging from 0-70oC.
- Prepare a series of test tubes containing the same volume of water (e.g. 10 cm3). Place the tubes in different water for five minutes.
- Place a single sample of beetroot into each of the eight test tubes. Leave for 15 minutes.
- Use forceps to remove the pieces of beetroot from each tube. Keep the coloured liquid and transfer into a cuvette.
- Use a colorimeter to measure how much light is absorbed by each liquid. The darker the solution (i.e. the more permeable the membrane), the more light is absorbed.
- Draw a graph plotting absorbance against temperature.
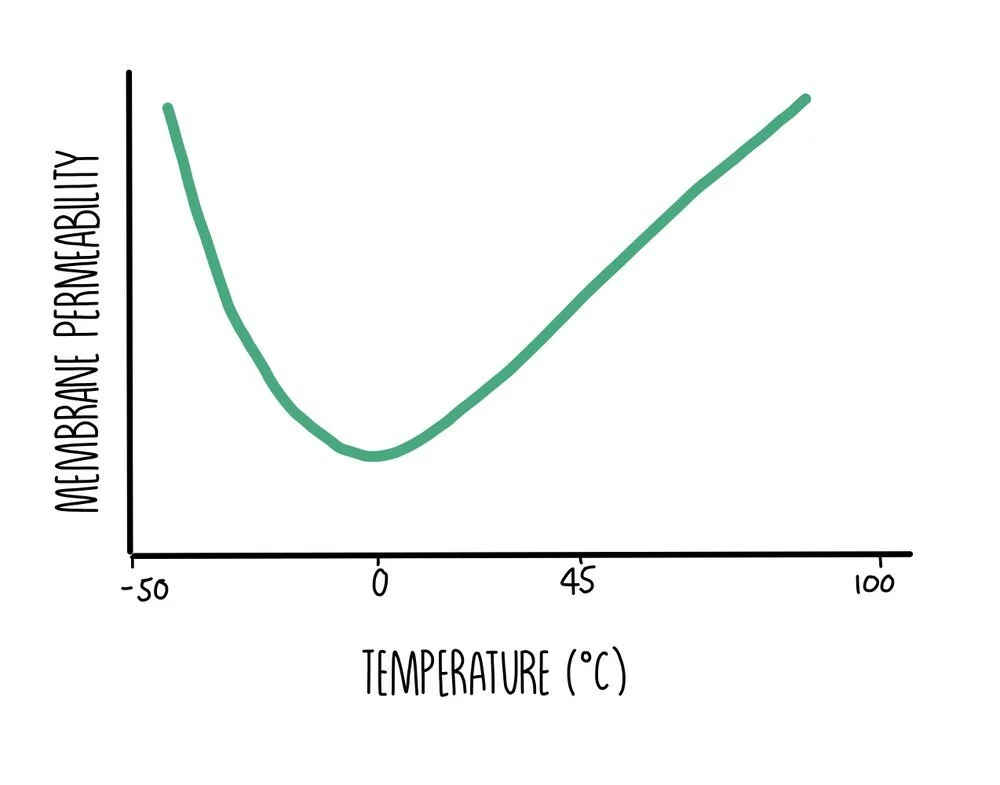
Temperature and membrane permeability
- At temperatures below freezing, the permeability of cell membranes increases since the proteins in the membrane unfold and become deformed. The molecules in the membrane have low amounts of energy so cannot move around much. The phospholipids become closely packed together which makes the membrane rigid. When temperatures fall low enough for ice crystals to form, these can puncture the membrane which increases its permeability when the cell membrane re-thaws, damaging the cell.
- Between temperatures of 0oC and 45oC, membranes are partially permeable. As temperature increases, the components in the membrane gain kinetic energy and move around more. The more fluid the membrane is, the more substances it allows through.
- As temperatures exceed 45oC, permeability increases rapidly because proteins in the membrane become denatured and start to unravel. In addition, water inside the cell cytoplasm expands, putting pressure on the cell membrane and creating gaps within the bilayer.
Transport across cell membranes
Molecules can make their way across the plasma membrane in one of three ways: osmosis (if the molecule is water), diffusion (if it is a molecule moving down its concentration gradient) or active transport (if it is a molecule moving against its concentration gradient. For larger substances to get into or out of the cell, such as proteins or carbohydrates, they will rely on processes called endocytosis and exocytosis.
Simple diffusion
Diffusion is the movement of molecules down their concentration gradients. When molecules move by simple diffusion, they pass directly through the phospholipid bilayer. It is a passive process which means that no energy is required. Oxygen and carbon dioxide move by simple diffusion when they pass from the alveoli into the bloodstream during gas exchange.
The rate of simple diffusion depends on:
The concentration gradient – the steeper the gradient, the faster the rate of diffusion
The thickness of the exchange surface – thicker exchange surfaces mean a longer diffusion distance
Surface area – larger surface areas mean more space for diffusion to take place. Adaptations like microvilli in the small intestine increase surface area.
Facilitated diffusion
Facilitated diffusion involves the movement of molecules down their concentration gradients. It differs from simple diffusion in the fact that a carrier protein or a channel protein within the cell membrane helps them get from one side to the other. This is also a passive process. An example of facilitated diffusion is the movement of glucose molecules into liver cells through glucose transporter proteins embedded in the plasma membrane.
The rate of facilitated diffusion depends on:
The concentration gradient – the steeper the gradient, the faster the rate of diffusion
The number of channel/carrier proteins – the more transport proteins, the faster substances can be moved across the membrane
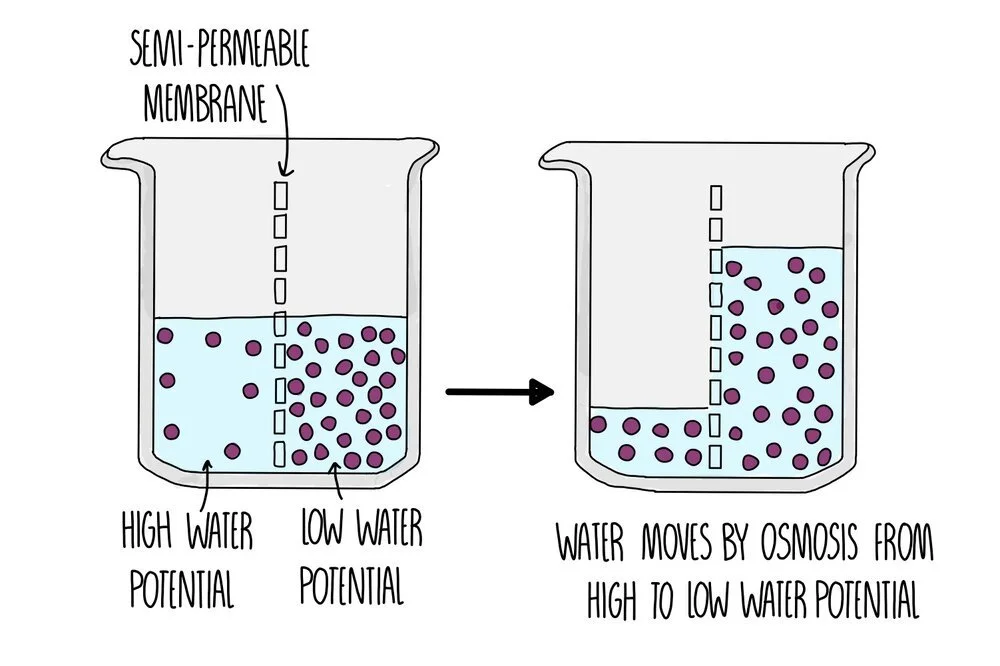
Osmosis
Osmosis is the movement of water molecules down its concentration gradient across a partially permeable membrane. It is a passive process so does not require energy in the form of ATP. Osmosis is responsible for the movement of water molecules into the root hair cells of plants, for example.
You can think of it as how ‘pure’ the water it. Pure water has the highest water potential – all other solutions will have lower water potentials in comparison, with the more solute dissolved in it, the lower the water potential. Two solutions with the same water potential are isotonic.
Rate of osmosis depends on:
The water potential gradient – the steeper the gradient, the faster the rate of osmosis
The surface area – the larger the surface area, the more space there is for osmosis to take place
The thickness of the exchange surface – thinner surfaces mean a shorter diffusion distance
Determining the water potential of potato cells
You can set up an experiment to determine the water potential inside potato cells. If potato is placed in a solution of higher water potential, water will move into the cells and the potato gains mass. If it is placed in a solution of lower water potential, it will lose mass. When there is no change in mass, the solution and the potato’s cytoplasm are isotonic and you have found the water potential inside the cells.
Method:
- Prepare cylinders of potato using a cork borer and cut into three.
- Measure the mass of the potato pieces using a mass balance.
- Prepare serial dilutions of sucrose solution. First, make a 2M solution with a volume of 10 cm3. Transfer half (5 cm3) to another test tube, with an equal volume of water. Repeat three more times so that you end up with solutions of 2M, 1M, 0.5M, 0.25M and 0.125M.
- Place the potato pieces in each solution and leave for 30 minutes.
- Remove from solution and dry with a paper towel.
- Record the mass of each potato piece and calculate the percentage change in mass.
- Draw a graph with percentage change in mass on the y-axis and concentration of sucrose solution on the x-axis. Read off the sucrose concentration where the change in mass = 0. Look up the water potential for this sucrose concentration – it’s the same as the water potential of the potato cells.
Active transport
Active transport moves molecules against their gradient, from low to high concentration.
This involves a carrier protein which carries the molecule from one side of the membrane to the other.
It is an active process and uses ATP to release energy.
An example is the transport of glucose from the villi of the intestine into the bloodstream.
Cotransporters are a special type of carrier protein which can bind to two molecules at a time. The concentration gradient of one molecule is used to transport the other molecule against its gradient.
Rate of active transport depends on:
The number of carrier proteins in the membrane – more proteins means faster rate of transport
The speed of each carrier protein – the faster carrier proteins work, the faster the rate of active transport
The rate of respiration – the faster respiration is taking place, the more ATP is generated. Inhibition of respiration will also inhibit active transport
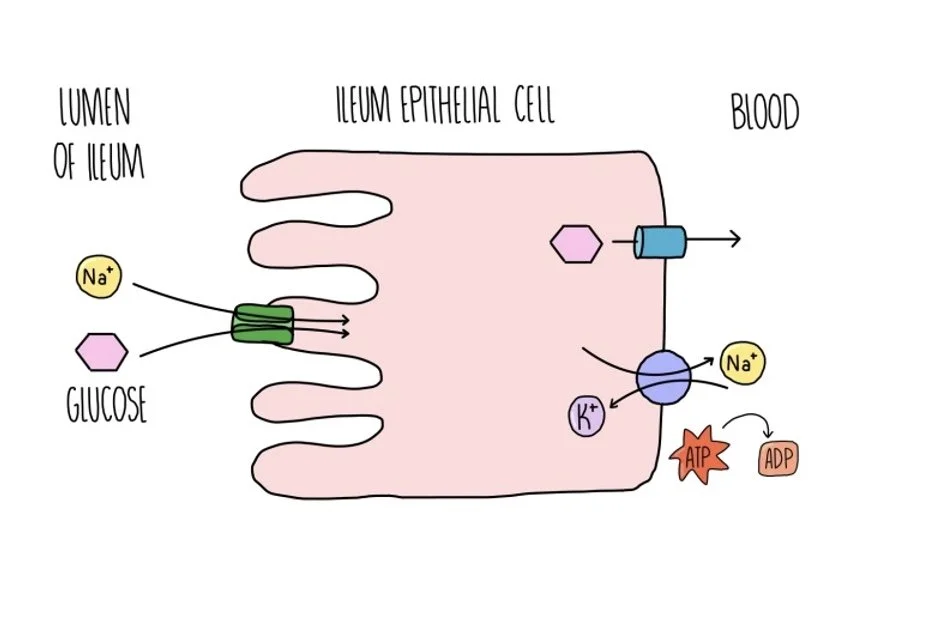
Cotransport of glucose and sodium ions in the intestine
Inside our intestine, carbohydrates are digested into glucose which needs to be absorbed into the bloodstream. To maximise the number of glucose molecules taken into our body, the gut relies on active transport. In the epithelial cells lining the ileum, a sodium-potassium ion pump actively transports sodium ions out of the cell, creating a sodium ion gradient between the ileum cell and the intestinal lumen. A co-transporter protein uses the sodium ion gradient to transport glucose against its gradient. It picks up both molecules on the lumen side and transports them both into the ileum cell. Glucose can then move into the bloodstream by facilitated diffusion, through a glucose channel.