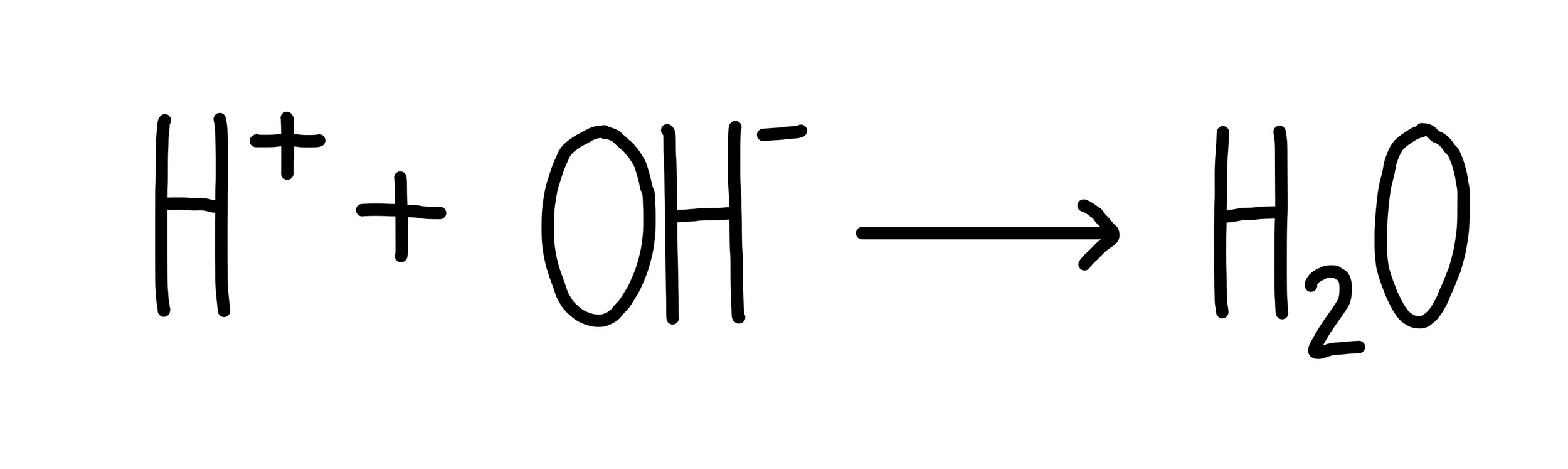Compounds, Formulae and Equations
Charges of ions
You can predict the charge of certain ions based on their group in the periodic table:
The ions that are formed from atoms in the transition metal block of the Periodic Table, or ions formed from molecules, are included in the table below:
Writing formulae for ionic compounds
When two ions bind to form a compound, their charges cancel. For example, one compound of magnesium iodide needs two iodide ions to neutralise the 2+ charge on the magnesium ion. Use the crossing-over method to help you.
Balancing equations
Chemical reactions need to have the same number of atoms on each side of the equation. For the reaction between sodium hydroxide and sulphuric acid, start by writing out a list of how many atoms there are on each side of the equation.
The little numbers written after an atom mean that we have to multiply that atom by the little number that follows it. Only the larger numbers in front mean that we have to multiply everything in the molecule by that number.
Na = 1 Na = 2
O = 5 O = 5
H = 2 H = 2
S = 1 S = 1
The only thing that is unbalanced are the sodium atoms. To make these equal on both sides of the equation, add a big number 2 in front of the NaOH.
Notice that by writing a ‘2’ in front of NaOH, I have also increased the numbers of oxygen and hydrogen atoms. My tally now stands as:
Na = 2 Na = 2
O = 6 O = 5
H = 4 H = 2
S = 1 S = 1
I have one more oxygen and two more hydrogens on the left hand side compared to the right. To balance this, I can add a big number 2 in front of the H2O and then everything is equal.
State symbols
State symbols provide information about the state of matter of a substance. They include:
Solid (s) – any metal (except mercury), metal oxides and metal carbonates will be solid at room temperature.
Liquid (l) – water or anything molten will be a liquid.
Aqueous (aq) – anything that is in solution. If a soluble compound is being formed, it will be aqueous. All acids and alkalis will be aqueous.
Gas (g) – most non-metals are gaseous at room temperature
Adding state symbols to the reaction described above will give us:
Ionic equations
Ionic equations are a way of simplifying equations down to the reacting particles. To write an ionic equation, separate out the aqueous molecules into the ions that make them up. Anything that is solid, liquid or gaseous is written out as normal. In our example of the reaction between sodium hydroxide and sulfuric acid, I’d write out the ionic equation as:
Anything that is the same on both sides is cancelled out. These ions are present in the reaction mixture, but since they don’t change they’re not actually involved in the reaction. They’re called spectator ions.
After cancelling out the spectator ions, I’m left with:
I can simplify this even further by removing the coefficients in front of each substance, as they are all the same. This gives: