Crude Oil and Hydrocarbons
Crude oil is a fossil fuel containing a mixture of hydrocarbons. After separation by fractional distillation, the shorter hydrocarbons are used for petrol because they release loads of energy when burned. Longer hydrocarbons can be converted into shorter ones by cracking.
Crude oil, hydrocarbons and alkanes
Crude oil is formed from the remains of living material which has been compressed under high pressures over millions of years. It is found deep under rocks and can be burned to release energy. It is known as a fossil fuel and is finite, which means there is only a limited amount underground and will eventually run out.
Crude oil consists of a mixture of hydrocarbons, which are compounds made up of only carbon and hydrogen atoms. Short hydrocarbons burn very efficiently so are useful compounds for the production of diesel and petrol.
Most hydrocarbons found in crude oil are alkanes. Alkanes contain just single carbon bonds and have the general formula CnH2n+2. They make up something called a homologous series, which is a group of molecules which share the same general formula, undergo the same chemical reactions and show trends in their physical properties. The first member in the alkane homologous series is methane, which consists of one carbon connected to four hydrogen atoms. The second member of the series is ethane, which is made up of two carbon atoms connected to six hydrogens.
The image below shows the first six members in the homologous series on alkanes. You can see that each alkane is the same as the last, but has an extra CH2 group attached to the chain.
Fractional distillation
Fractional distillation is used to separate crude oil into fractions, containing a group of hydrocarbons of similar lengths. The crude oil is vaporised and passed into a fractionating column which is hot at the bottom and cooler at the top. Vapours from the oil rise through the column and condense when the temperature of their boiling point is reached. Longer hydrocarbons have higher boiling points than shorter hydrocarbons because they form stronger intermolecular forces between hydrocarbon molecules.
In the 20th and 21st centuries, we have come to rely on the compounds in crude oil for things like petrol and diesel but also for making plastics, solvents and detergents. The extraction of huge amounts of crude oil has had a devastating effect on the environment, releasing large amounts of carbon dioxide into the atmosphere and depleting crude oil resources.
Properties of hydrocarbons
Longer hydrocarbons:
Have higher boiling points (condense towards the bottom of the column)
Are less volatile (do not evaporate easily)
Are less flammable (do not ignite easily)
Are more viscous (do not flow easily and have a thick, treacly appearance)
Have a darker colour
Shorter hydrocarbons:
Have lower boiling points (condense at the top of the column)
Are volatile (evaporate easily)
Are flammable (ignite easily)
Are less viscous (they flow easily)
Are either colourless or pale in colour
These properties make short-chain hydrocarbons really useful for car fuels, which means there is a much bigger demand for the shorter hydrocarbons compared to the less flammable long chain hydrocarbons. Since crude oil consists of a large proportion of the longer hydrocarbons, we use a process called cracking to snip longer hydrocarbons into shorter, more useful ones.
All hydrocarbons can be burnt to release heat energy, which is why they make good fuels. Remember that combustion is simply reacting the molecule with oxygen. The products of a combustion reaction are carbon dioxide and water.
Cracking and alkenes
Crude oil is rich in longer hydrocarbons and contains fewer shorter hydrocarbons. Since shorter hydrocarbon compounds burn more efficiently and make better fuels, there is more demand for the shorter hydrocarbons than the longer ones. Cracking is a processed used to convert long-chain alkanes into shorter alkanes and alkenes. Temperatures of 600-700oC and a zeolite catalyst are used. Zeolite is a crystal made up of silicon, aluminium and oxygen.
Shorter saturated hydrocarbons (alkanes) are mostly used for fuels. Alkenes can be used in the production of plastics in polymerisation reactions.
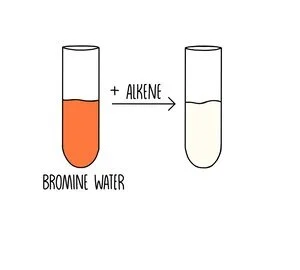
Alkenes are more reactive than alkanes because they contain a double carbon bond. For instance, alkenes can undergo addition reactions with bromine, whereas alkanes cannot react with bromine under normal conditions. The double bond breaks and bromine is added to the carbon atoms which made up the double bond. Bromine water is orange when unbound to another molecule and loses its colour when bound to the hydrocarbon. The orange to colourless colour change can be used as a test to detect the presence of an unsaturated compound.
This is called an addition reaction because the double carbon bonds breaks apart and the bromine atoms are added to the carbons.
Did you know…
During the coronavirus pandemic and national lockdowns, the price of crude oil fell to a negative value for the first time in history. There was a dramatic drop in demand as international flights were grounded and people stayed indoors. It means that oil companies had to pay others to take it off their hands, renting out huge spaces to store unused tanks of crude oil.
Next Page: Alkenes and Alcohol