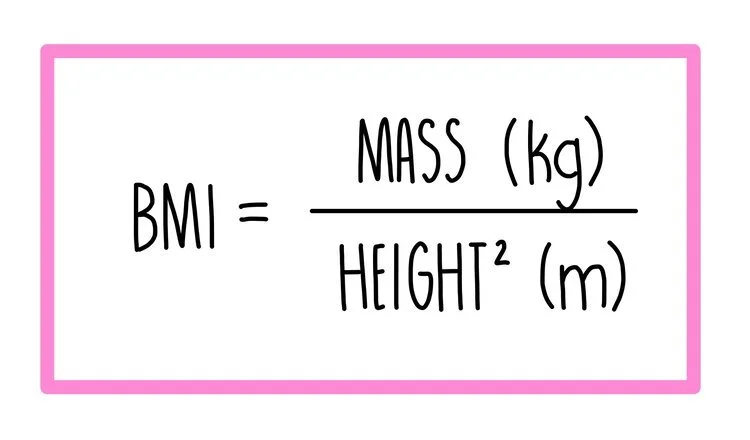Diet and Health
Energy budgets and diet
An individual’s ‘energy budget’ refers to the amount of energy taken in (i.e. calories consumed) and the amount of energy used. If someone’s energy intake is higher than their energy use, the excess energy is converted to fat and they will put on weight. If their energy intake is less than their energy use, they will burn fat reserves and lose weight.
Investigating vitamin C content of food
You can investigate how much vitamin C a food sample contains using the molecule DCPIP, which changes from blue to colourless is the presence of vitamin C. You’ll need to follow the method below:
Create a 1 mg cm-3 vitamin C solution. Prepare serial dilutions of 0.5, 0.25, 0.125 and 0.0625 mgcm-3
Measure out a set volume of DCPIP using a measuring cylinder and pour into a test tube.
Dropwise, add vitamin C solution to the test tube and gently shake for 30 seconds. Keep adding drops of the vitamin solution and shaking until the blue colour disappears.
Record the number of drops added to cause the colour change.
Repeat twice more with each vitamin C concentration and calculate a mean.
Repeat with serial dilutions.
Draw a graph showing volume of vitamin C solution against concentration and connect data points with a curve of best fit. This is known as a calibration curve.
Now, test your unknown solution using the method above and use the calibration curve to determine its concentration.
Control variables: temperature, volume of DCPIP, time spent shaking the solution.
Carbohydrates
Carbohydrates are long chains of sugar molecules (the technical term is ‘polysaccharides’) formed from lots of individual sugar molecules (monosaccharides) joining together. When two monosaccharides react to form a disaccharide, a condensation reaction occurs. During the condensation reaction, a molecule of water is removed (from a hydroxyl group on one sugar and a hydrogen on another) and a glycosidic bond forms. When polysaccharides are broken down during digestion, a hydrolysis reaction occurs in which a water molecule is added to break the glycosidic bond.
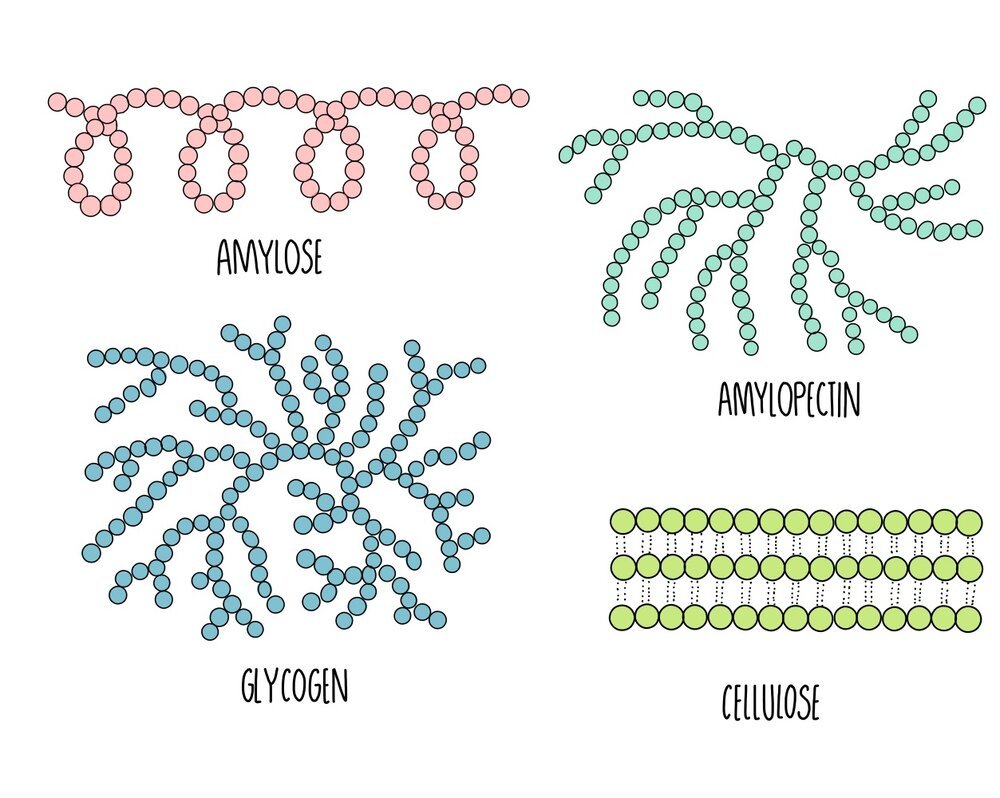
The polysaccharides found in plants are starch and cellulose. Starch is broken down by the plant when it needs energy and cellulose is the major component of plant cell walls. Starch exists in two different forms: amylose and amylopectin. In animals, the energy storage carbohydrate is glycogen.
Amylose: unbranched spiralling chains of alpha-glucose molecules. Its coiled structure means that it is very compact so lots of amylose can be packed into a cell.
Amylopectin: branched chains of alpha-glucose molecules. Its branches increase its surface area which means that enzyme can quickly break it apart when glucose is needed for respiration.
Cellulose: long unbranched chains of beta-glucose molecules. Multiple chains are linked together by hydrogen bonding to formstrong structures called micofibrils. The strong microfibrils in the cell wall help to give plant cells their shape and structural support.
Glycogen: branched chains of alpha glucose, similar to amylopectin but with more side-branches. This gives it a large surface area for enzyme action to release glucose when energy is needed. It is more compact that amylopectin, which means more can be stored in a cell.
Lipids
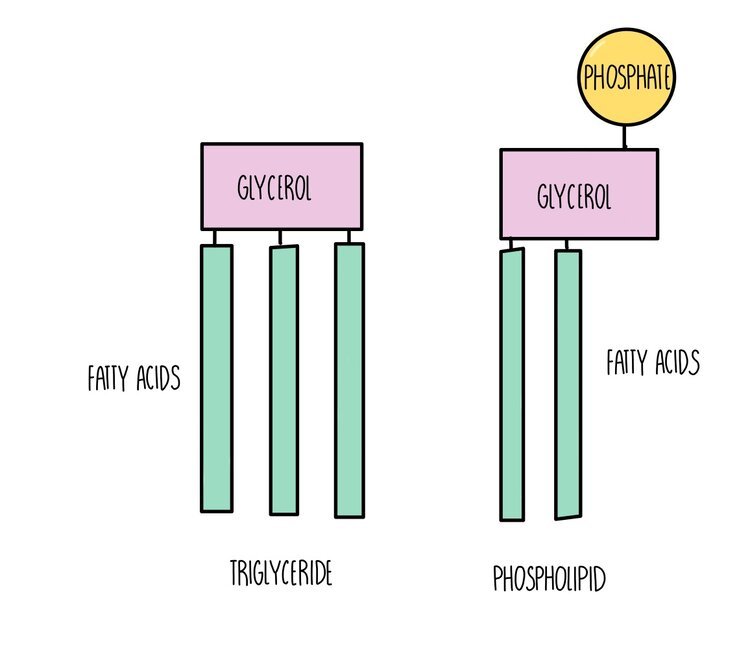
Triglycerides, phospholipids and cholesterol are all types of lipid. Triglycerides and phospholipids have similar structures as they are both made up of glycerol connected to fatty acids molecules through ester bonds. However, triglycerides contains three fatty acid ‘tails’ whereas phospholipids only have two. Phospholipids also possess a phosphate group which is absent in triglycerides. The fatty acid tails of triglycerides or phospholipids can be saturated (only single carbon-carbon bonds) or unsaturated (contain at least one double carbon bond).
A diet high in saturated fat increases the risk of CVD because it increases blood cholesterol levels.
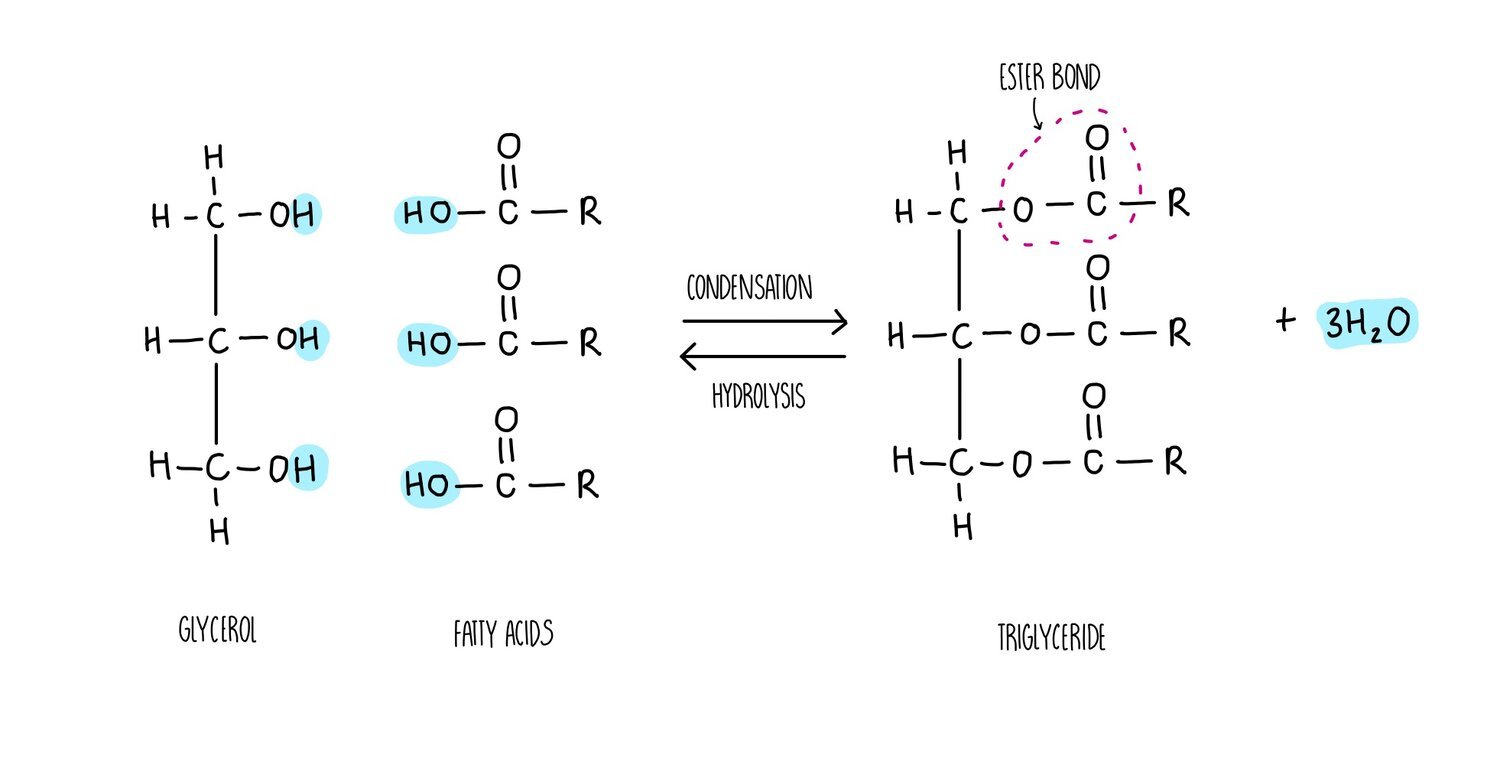
The synthesis of a phospholipid or triglyceride from glycerol and fatty acids involves the formation of an ester bond. Just like we saw for the formation of a glycosidic bond, the synthesis of ester bonds is a condensation reaction in which a water molecule is released. Breaking apart a triglyceride or phospholipid involves the addition of water in a hydrolysis reaction.
Different types of lipids have different functions in living organisms:
Triglycerides: used as an energy store. A lot of energy is released when the ester bonds are hydrolysed - around twice as much compared to the breakdown of carbohydrates. Inside cells, triglycerides group together into lipid droplets where the hydrophobic tails face inwards and the hydrophilic heads face outwards. These insoluble droplets make good energy storage molecules since they do not affect the osmotic potential of the cell.
Phospholipids are the main component of cell membranes. They form a phospholipid bilayer with the hydrophobic tails facing each other and the hydrophilic heads facing outwards, forming a barrier to prevent any polar molecules from entering or leaving the cell.
Cholesterol is also found in cell membranes and helps to strengthen the membrane. Cholesterol pushes the hydrophobic tails of phospholipids closer together, making the membrane more rigid.
Cholesterol
Not all fats are created equally - different types of fat can have very different effects on our bodies. Saturated fats, which contain only single C-C bonds, are unhealthier and are found in animal-based foods such as cheeses, butter and beef burgers. Unsaturated fats, which contain at least one double C=C bond, are healthier and are typically found in plant-based foods such as olive oil, nuts and oily fish.
Saturated fats increase the levels of a molecule called ‘low-density lipoprotein (LDL)’ in the blood. Lipoproteins are like little packages of lipids surrounded by protein and function to transfer cholesterol between the liver (where it is made and broken down) and the body tissues. LDLs take cholesterol from the liver and deposit it in the blood, where it will circulate until it is needed by our cells. The other type of lipoprotein, called high-density lipoprotein (HDL) transports cholesterol from the bloodstream and returns it to the liver where it can be destroyed. A high intake of unsaturated fats increases HDL levels in our blood. This means that a high amount of LDLs and low levels of HDLs can have the effect of increasing cholesterol levels, which make us more susceptible to cardiovascular disease.
Our cells need cholesterol because it is used to make sex-hormones (such as oestrogen), to synthesise bile and as a component of plasma membranes. The problem is that our liver already synthesises enough cholesterol, then on top of that we consume way more than our bodies know what to do with. Therefore, it is important that we have a high ratio of HDL to LDLs by eating more unsaturated fats to saturated fats. High levels of HDLs will ensure that excess cholesterol is taken back to the liver to be broken down and removed from our body.
Obesity Indicators
Doctors use measurements such as the body mass index (BMI) and waist-to-hip ratio to determine whether someone is packing on the pounds and needs to lay off the biscuits. People who are identified in the overweight and obese categories will be more at risk of developing CVD and will be advised by their doctor to eat healthier and to carry out more exercise.
Body mass index (BMI) - a person’s BMI is calculated by dividing their body mass by their height squared. The problem with calculating a BMI is that it doesn’t take into account whether their body mass consists of fat or muscle. Using BMI, a bodybuilder could be classified as morbidly obese even though they have little fat on their body and are perfectly healthy.
Waist-to-hip ratio - the waist-to-hip ratio is calculated by dividing an individual’s waist size by their hip size. It takes into account where fat is accumulating on the body and can be a more accurate prediction of whether a person has excess weight.