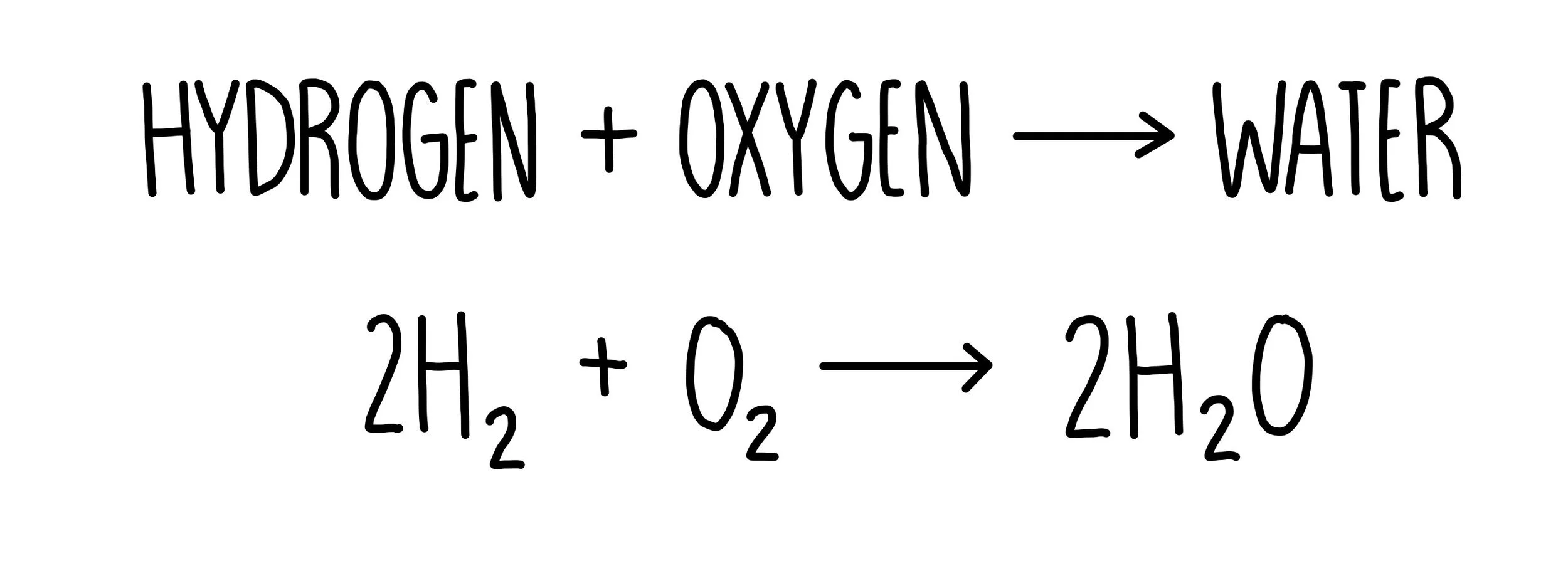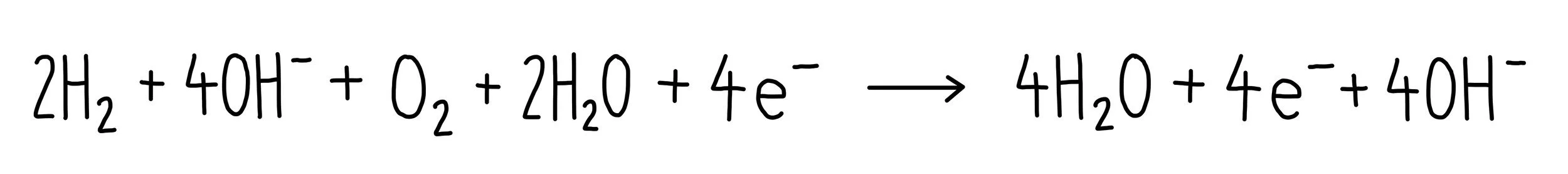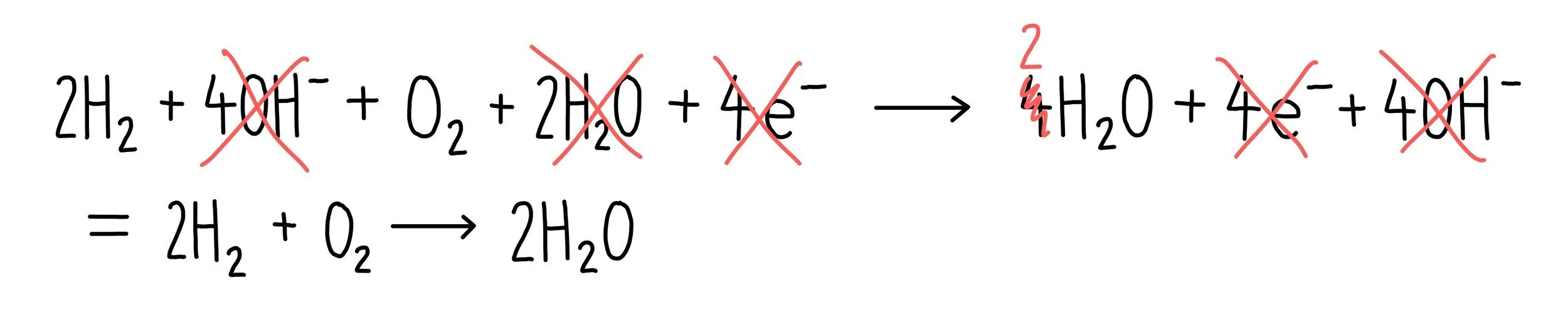Electrolysis and Fuel Cells
Electrolysis is the process by which ionic compounds are broken down into simpler substances when an electric current is passed through them. It can be used to purify a metal, such as aluminium, from its ore.
Electrodes
The electrolysis set-up consists of two electrodes: a positive electrode called the anode and a negative electrode called the cathode. You know that an ionic compound consists of positive and negative ions. When an ionic compound is dissolved in water or molten, these ions move apart and can move through the liquid. The positive ions (called cations) will be attracted towards the negative electrode (the cathode). The reason the cathode is negative is because an electric current is running from the anode towards the cathode, causing a build-up of electrons on the cathode. A positively charged ion will happily take an electron (or two) from the cathode to become neutral. On the other hand, a negatively charged ion (an anion) will deposit an electron, or two, onto the positive anode to become neutral. This is summarised below:
Anode: the positive electrode attracts negatively charged ions (anions), which will lose electrons to become oxidised
Cathode: the negative electrode attracts positively charged ions (cations) which will gain electrons and become reduced.
Remember OILRIG: oxidation is loss, reduction is gain.
Aqueous solutions
An aqueous ionic solution is simply where we have an ionic compound dissolved in water. The solution will contain the following ions:
- The ions which make up the ionic compound (such as sodium ions (Na+) and chloride ions (Cl-).
- The ions which make up water: hydrogen ions (H+) and hydroxide ions (OH-).
At the cathode
- Hydrogen (from the H+ in water) is produced unless the positive ion from the ionic compound is less reactive than hydrogen.
- If the metal ion is less reactive, this will be produced instead. The only metals less reactive are copper, silver, gold and platinum.
At the anode
- Oxygen (from the OH- in water) is produced unless the negative ion is one of the halogens.
- Remember that the halogens are what we call group 7 of the Periodic Table and include the elements fluorine, chlorine, bromine and iodine.
- If there are halide ions, the halogen will be produced instead of oxygen.
Example: aqueous sodium chloride solution
- We know that at the cathode (negative electrode), either hydrogen (from H+) or sodium (Na+) will be produced. The rule is that it will be the least reactive of the two elements. In this case, hydrogen is the least reactive so hydrogen gas will form at the anode.
- We know that at the anode (positive electrode), oxygen is formed in the absence of a group 7 element. Here, chloride ions are in our solution, which is a group 7 element so chlorine gas forms at the cathode instead of oxygen.
Molten compounds
Molten ionic compounds are ionic substances which have been melted into a liquid form. They are not dissolved in water, so H+ and OH- ions are not present. This means that hydrogen and oxygen cannot form at the anode and cathode.
Let's say we have the molten form of lead bromide. This means that we have a liquid consisting only of lead ions (Pb2+) and bromide ions (Br-). Because we only have one positive ion to gain electrons at the anode and one negative ion to give away electrons at the cathode, it's pretty obvious what is formed:
- Pb2+ moves towards the cathode and solid lead (Pb) is produced.
- Br- moves towards the anode and bromine gas (Br2) is produced.
Half equations
We can represent the gain or loss of electrons during electrolysis using half equations. Remember that the electrons should be written on the left hand side of the equation when they are gained by an ion (reduction) and on the right when being lost (oxidation). A good trick to make sure you’ve written the half equation properly is to make sure the charges on the left and right hand sides are equal.
Cells and batteries
A cell is the proper word for what you probably think of as a battery – the cylindrical objects that power TV remotes and other devices. Cells contain chemicals in the form of a solid metal (electrode) and an ionic solution (the electrolyte), which react and generate electricity by releasing electrons. The voltage produced by a cell depends on the type of electrode and electrolyte used. You can make a simple cell by connecting two different metals in contact with an electrolyte solution.
A battery is two cells joined together in series to increase the voltage. Batteries can be rechargeable or non-rechargeable. In non-rechargeable cells or batteries (such as alkaline batteries), the reactants will eventually be used up and the reaction will stop. With rechargeable cells and batteries, an external electric current reverses the reaction once the reactants have been used up. This ensures that the reaction can keep going and the battery can continue to supply electricity.
Fuel cells
Fuel cells differ from chemical cells in that they generate a continuous voltage, providing they have a fuel supply (such as hydrogen) and oxygen. Within the fuel cell, the fuel is oxidised electrochemically rather than being burned. This means that the reaction takes place at a lower temperature than a typical combustion reaction. Energy is released as electrical energy.
The overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water.
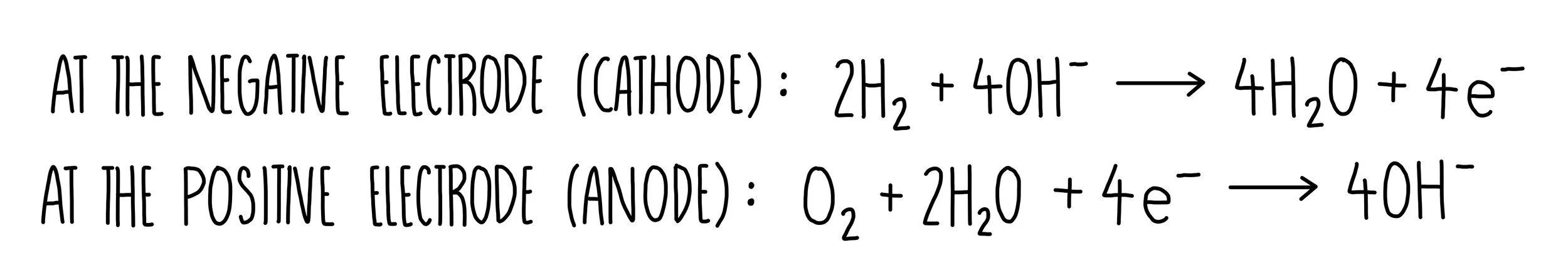
We can break down this overall reaction into two half equations, one of which involves oxidation at the anode and the other involves reduction at the cathode.
Adding these two half equations together, we get:
To get the final equation, we need to cancel out anything that is the same on both sides. In this case, this includes the hydroxide ions, electrons and two water molecules. This results in the following overall equation:
Hydrogen fuel cells are a potential alternative to rechargeable cells and batteries. The advantages of hydrogen fuel cells are that they only produce water as a product (so no toxic products formed). They are also small and easy to maintain. However, they are very expensive to make and require a constant supply of hydrogen gas which is flammable and difficult to store. Non-rechargeable alkaline batteries are cheaper to make but they may accumulate in landfill sites once they have run out. These batteries are recyclable but the recycling process is expensive.