Energetics: Answers
Are combustion reactions endothermic or exothermic? Why?
Combustion reactions are exothermic because they release heat energy to the surroundings.
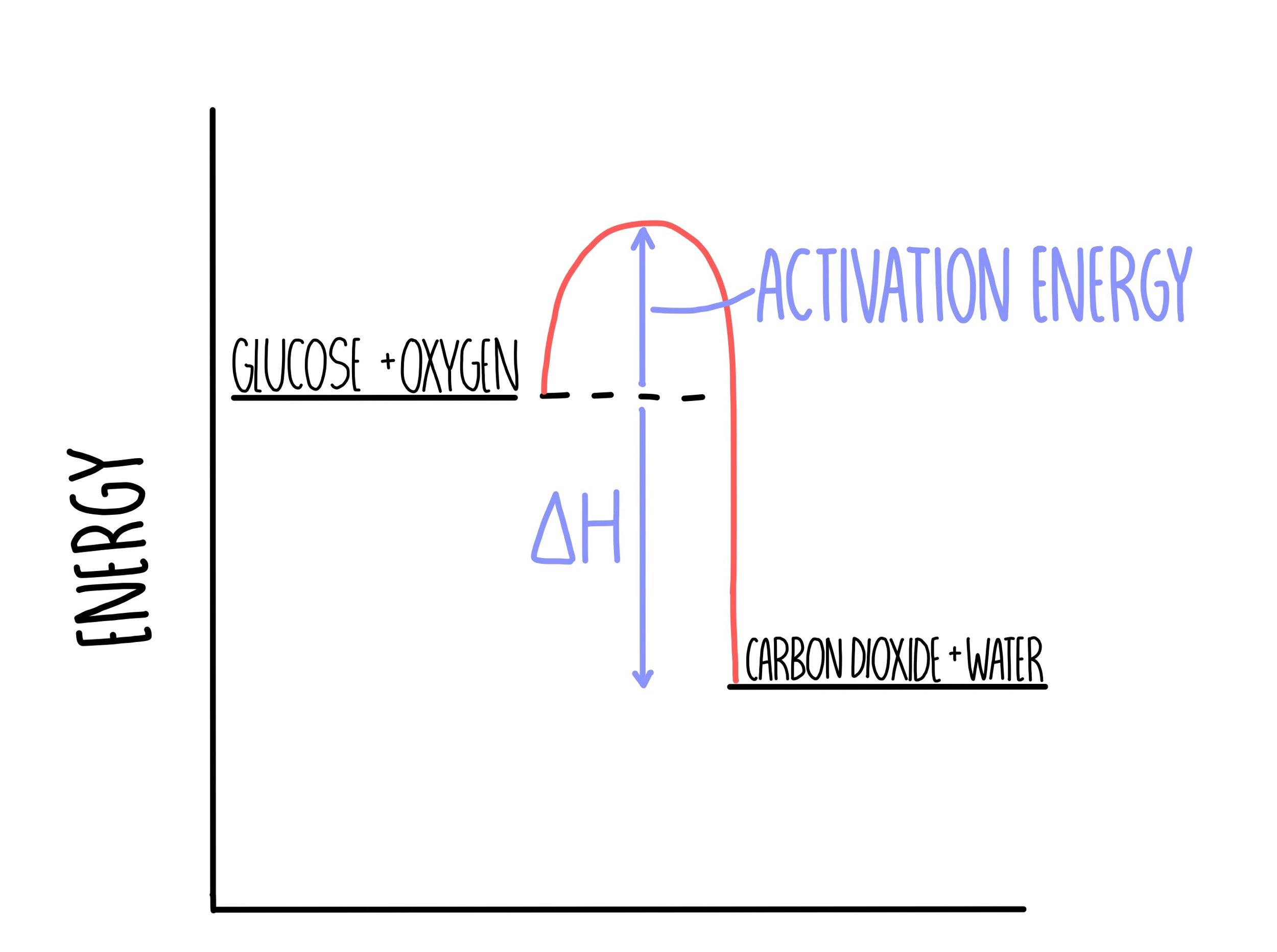
Draw a reaction profile diagram for the combustion of glucose with oxygen to form carbon dioxide and water. Include arrows and labels for activation energy and enthalpy change.
5 g of propanol is burnt underneath 100 g of water, which raises the temperature of water from 20 to 86 degrees Celcius. Specific heat capacity = 4.2 J/K/g.
Calculate the heat energy (Q) in kJ released by the propanol.
Q = mass (of water) x specific heat capacity x temperature change
Q = 100 x 4.2 x 66 = 27 720 J
Q = 27 720 / 1000 = 27.720 kJ
Calculate the molar enthalpy change in kJ/mol for the reaction.
Molar enthalpy change = Q / moles
Moles = mass / Mr
Moles = 2 / 60 = 0.03
Molar enthalpy change = 27.720/0.03 = 924 kJ/mol
Why might the enthalpy value recorded in this experiment differ from that found in a data book? What can we do to make our data more accurate?
Not all the heat burnt will be transferred to the water. Instead, some will be lost to the surroundings or will be absorbed by the equipment. To make our data more accurate, we can insulate the can of water and add a lid.