Enzymes
Enzymes are proteins which speed up reactions in living organisms. They’re really useful because they allow reactions which would be really sluggish happen in a matter of milliseconds. They also allow reactions to happen at a lower temperature so they can take place at body temperature. There are two ways scientists think enzymes work - the ‘lock-and-key’ model and ‘induced-fit’ model.
Enzyme function and structure
Enzymes are biological catalysts - they speed up the rate of chemical reactions happening inside our body. They work by reducing the activation energy of a reaction. Activation energy is defined as the minimum amount of energy needed for a reaction to happen. If less energy is needed, then reactions can take place as lower temperatures than would be needed without an enzyme. Without the enzymes in our bodies, the reactions that happen inside of us would not be possible at normal body temperature. Remember that enzymes are unchanged at the end of a reaction which means they can be reused.
Enzymes can be classed as either intracellular if they catalyse reactions inside cells e.g. RNA polymerase, or extracellular if they catalyse reactions outside of cells e.g. amylase. All enzymes are globular proteins and have regions called active sites. The active site of an enzyme has a specific shape and allows the substrate to bind. Other enzymes may have regulatory regions where an inhibitor can bind, which we refer to as the allosteric site.
Mechanisms of enzyme action
Scientists have two ideas to explain the way in which enzymes work: the ‘lock-and-key’ model and the ‘induced-fit’ model. They are models because they are our best-accepted theories based on the evidence we have available.
Lock and Key model
The lock and key model is the simpler of the two theories of enzyme action. This model suggests that the substrate fits into the enzyme’s active site in the same way in which a key fits into a lock. The shape of the substrate and the active site are perfectly complementary to each other. Catalysis happens in the following stages:
The substrate binds to the enzyme’s active site, forming an enzyme-substrate complex (ES complex).
The enzyme converts the substrate into product, forming an enzyme-product complex (EP complex).
The product is released from the enzyme’s active site.
The Induced Fit model
The induced fit model suggests that the shapes of the enzyme’s active site and its substrate are not exactly complementary, but when the substrate enters the active site, a conformational change (change of shape) occurs which induces catalysis. The induced fit model can be broken down into the following stages:
The substrate enters the enzyme’s active site, forming an ES complex.
The enzyme undergoes a conformational change which causes the conversion of substrate into product, forming an EP complex.
The product is released from the enzymes active site.
Comparing the two models of enzyme action
The advantage of the lock-and-key model is that it explains why most enzymes display such high specificity to their substrates. Each enzyme will catalyse only a certain type of reaction and will only bind to a single specific substrate out of the millions of different molecules that are floating around our bodies. However, not all enzymes catalyse a single chemical reaction. For example, lipase exhibits broader specificity and can bind to a variety of lipids, which only the induced fit model is able to explain. In addition, the induced fit model is better able to explain how catalysis actually occurs. A conformational change, which would place stress on the bonds within the substrate can explain how bonds would break in order for the products to form. This makes the induced fit model the more widely accepted model of the two.
Factors which affect rate of reaction
Enzyme concentration
As enzyme concentration increases, the rate of reaction increases since more active sites will be available to bind to substrate molecules. This means that there will be more frequent collisions between the enzyme and substrate, so there will be more formation of enzyme-substrate complexes. However, a point will be reached when increasing enzyme concentration does not result in further increases in reaction rate. At this point, something else has become a limiting factor, such as the availability of substrate.
Substrate concentration
As substrate concentration increases, the rate of reaction increases since there are more substrate molecules to fill the enzyme’s active sites. There will be more frequent collisions so more formation of ES complexes. At some point a ‘saturation’ point is reached where all of the enzyme’s active sites are occupied with substrate molecules, so the addition of more substrate molecules will have no effect on the rate of reaction. At this point, the reaction is proceeding as fast as possible, which is referred to as Vmax. The only way the reaction can go any faster is by increasing enzyme concentration.
Temperature
At low temperatures, the rate of reaction will be slow because the enzyme and substrate have low amounts of kinetic energy. This means that there won’t be many collisions so there will be reduced formation of ES complexes. As the temperature is increased, the number of collisions increases, increasing the formation of ES complexes and increasing the rate of reaction. If the temperature becomes really high, hydrogen bonds will begin to break within the protein, causing it to unravel and become denatured. If enzymes are denatured, they lose the shape of their active sites which means they cannot bind to their substrate, decreasing the rate of reaction.
pH
Each enzyme has its own optimum pH at which it works best. Pepsin, the enzyme which digests protein in the stomach, works best in acidic environments whereas the enzymes responsible for the digestion of carbohydrates work better at a more neutral pH. Deviations from the optimum pH change the charge on the enzyme, which affects ionic bonding within its structure. Deviations in pH also break hydrogen bonds. This causes it to change shape and become denatured, decreasing the rate of reaction as pH deviates from the enzyme’s optimum conditions.
Competitive vs non-competitive inhibitors
Competitive inhibitors are ones which bind to the active site of the enzyme, blocking the substrate from binding - they compete with the substrate for access to the enzyme’s active site. The effect of a competitive inhibitor can be reduced by increasing substrate concentration. If there is way more substrate compared to inhibitor then the substrate is much more likely to collide with the enzyme’s active site.
Non-competitive inhibitors are ones which bind to a site on the enzyme away from its active site. This region is known as its allosteric site. The effect of a non-competitive inhibitor cannot be reduced by increasing substrate concentration.
Immobilised enzymes
Immobilised enzymes are enzymes which have been fixed in position by attaching to a static surface. It often improves the efficiency of an enzyme-catalysed reaction and means that the enzyme can be easily removed from the product after the reaction has taken place.
Immobilised enzymes are used in various industrial processes such as:
Medicine - immobilised enzymes are used to diagnose diseases and in pregnancy tests
Biofuels - enzymes can break down carbohydrates to produce ethanol-based fuels
Paper - immobilised enzymes are used in the wood-pulping process
Textiles - fibres are processed using immobilised enzymes
Food production - the manufacture of dairy products involves immobilised enzymes
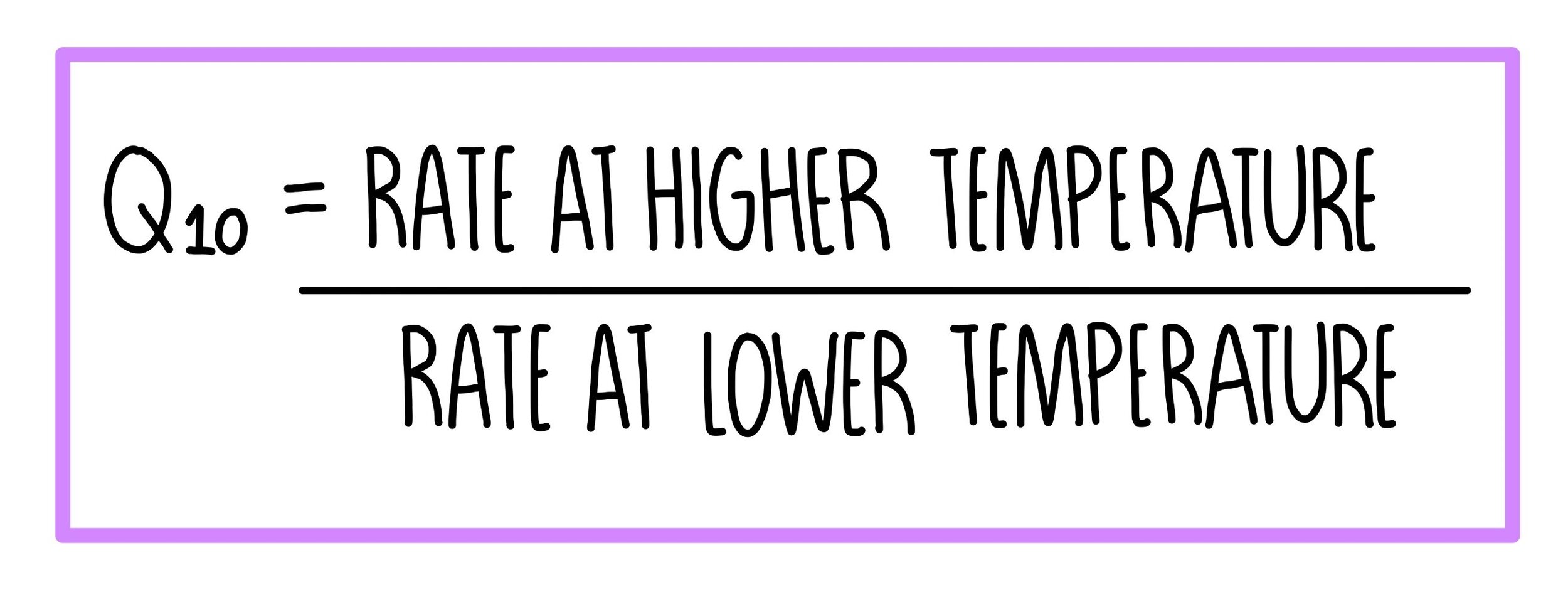
The temperature coefficient for an enzyme-controlled reaction
You may be asked to calculate the temperature coefficient, Q 10 , for an enzyme-controlled reaction. The coefficient shows how much the rate changes whenever the temperature of the reaction increases by 10 o C. It is calculated using the formula:
If a reaction has a Q 10 of 2, this means that the rate of reaction doubles when the temperature is increased by 10 oC. If it has a Q 10 of 3, the rate of reaction trebles when the temperature is increased by 10 oC. Most enzyme-controlled reactions will have a Q 10 of approximately 2.
Did you know..
Have you noticed that adding lime juice to guacamole can prevent it turning brown? Well, it’s all to do with enzymes. The low pH from the lime juice denatures the enzyme which is responsible for the browning process.
Next Page: Biological Membranes