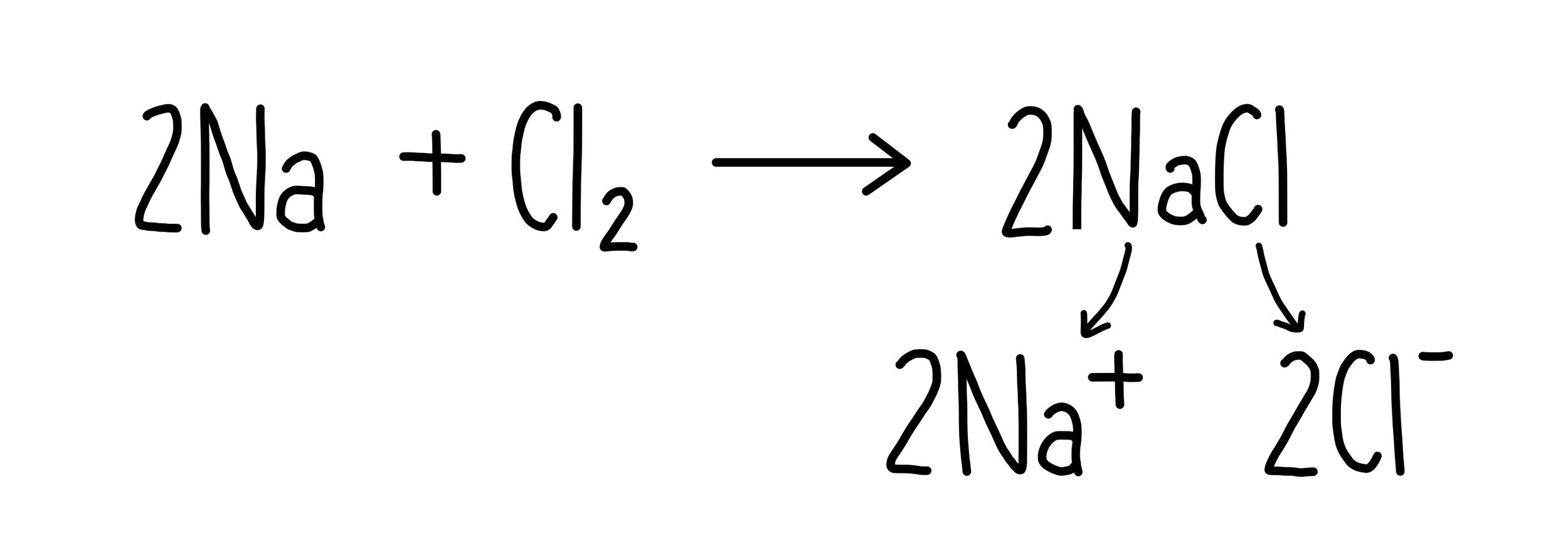Extraction and Uses of Metals
Metals are extracted from their ores using a method dependent on the position of the metal in the reactivity series. More reactive metals are extracted using electrolysis while less reactive metals can be heated with carbon. Other elements can be mixed with metals to produce alloys with different properties.
Extraction methods
Unreactive metals, such as silver and gold, can be found in the Earth’s crust in a pure form, uncombined to other elements. However, more reactive metals, such as aluminium and magnesium, will usually be found combined to another element in a compound. An ore is a rock that contains enough of the metal to make it economically worth extracting.
The method of extracting a metal depends on its position in the reactivity series.
If a metal is less reactive than carbon, it can be extracted by reacting it with carbon in a displacement reaction. In a displacement reaction, the more reactive metal is able to take the place of a less reactive metal in a compound, since the more reactive metal forms stronger bonds. Carbon replaces the less reactive metal in a redox reaction, where the carbon is oxidised and the metal is reduced. We therefore refer to this method as reduction using carbon.
For example, copper is below carbon in the reactivity series so it is extracted from copper oxide by reacting it with carbon, to form pure copper and carbon dioxide. The copper loses oxygen so it is reduced while the carbon gains oxygen so it is oxidised. That’s why we call this method reduction with carbon – because the element that we are purifying is reduced.
Elements which are more reactive than carbon will be extracted using electrolysis. Aluminium is more reactive than carbon so it must be extracted from ores containing aluminium oxide using this method. Electrolysis uses electricity to separate the metal from the other elements in the compound.
Oxidation and reduction (triple science)
We’ve seen that oxidation is the gain of oxygen and reduction is the loss of oxygen during a chemical reaction. We can also define oxidation as the loss of electrons and reduction as the gain of electrons that takes place during a reaction. You can use the anagram OILRIG to help you remember.
For example, let’s consider the reaction between sodium and chlorine to form sodium chloride.
Sodium loses an electron to form a positive sodium ion. It has been oxidised.
Chlorine gains an electron to form a negative chloride ion. It has been reduced.
Aluminium is used for aeroplanes because of its low density.
Uses of Metals
Aluminium: aluminium is a very lightweight metal so it is used as a material for aeroplanes and bicycles. It also has a protective outer layer of aluminium oxide, preventing it from corrosion. These properties make aluminium particularly useful for things like wrapping food and for drinks cans.
Copper: copper is a good conductor of heat and electricity so it is used in electrical wiring and heating systems.
Iron: pure iron is too soft and reactive to be a useful material so it is alloyed with carbon to make steel. Different amounts of carbon can be added to produce different properties:
Low carbon steel: this is malleable and can be easily shaped so is used as a material for cars
High carbon steel: this is very strong and inflexible - it is used as a material for bridges
Stainless steel: this is hard and resistant to corrosion so it used for things like cutlery and washing machines.
Alloys
An alloy is a mixture of two or more elements, where at least one element is a metal. Pure metals are arranged in a regular lattice where layers can slide over one another. The more difficult it is for the layers to move, the stronger the metal.
Different elements have different sized atoms so when you mix another element with a metal it disrupts the regular arrangement of atoms. The atoms are no longer aligned in neat rows which can easily slide over each other. This makes alloys much stronger and harder than pure metals.
Alloys may have additional useful properties that are absent in the pure metal. For example:
Aluminium alloys have a lower density than pure aluminium so are a useful material for aeroplanes.
Bronze is an alloy of copper and tin
Brass is an alloy of copper and zinc
The gold used in jewellery is usually an alloy of silver, copper and zinc. The amount of actual gold in gold jewellery is measured in carats. Pure gold is classed as 24 carat, whereas 50% gold is 12 carat.
Steel is an alloy of iron and carbon. The properties of steel can be altered by varying the proportion of carbon it contains. High carbon steel is strong but brittle, which means it can break easily when bent. Low carbon steel is malleable (can be easily shaped without breaking) but is soft. Stainless steels contain additional metals (chromium and nickel) which make them resistant to corrosion and useful for things like dishwashers and cutlery.
Aluminium is alloyed with other elements to make it more lightweight. Aluminium alloys are used for things like bikes, cars and aeroplanes.