Gas Exchange and Cell Membranes
Diffusion
Diffusion is the passive movement of molecules from an area of high concentration to an area of low concentration (i.e. down a concentration gradient).
When an equilibrium is reached on both sides of a membrane, diffusion is still taking place but there is no more net movement from one side to another.
Adaptations of exchange surfaces
For respiration, organisms need to take oxygen into their bodies and remove carbon dioxide. These gases diffuse across exchange surfaces, such as the lungs, so exchange surfaces are adapted to make diffusion as efficient as possible.
Most gas exchange surfaces are extremely thin (sometimes just one cell thick), ensuring a short diffusion pathway across the exchange surface. They will also have a large surface area to volume ratio which provides more space for the diffusion of gases. The organism itself will also have features which maximise the concentration gradient of gases across the exchange surface.
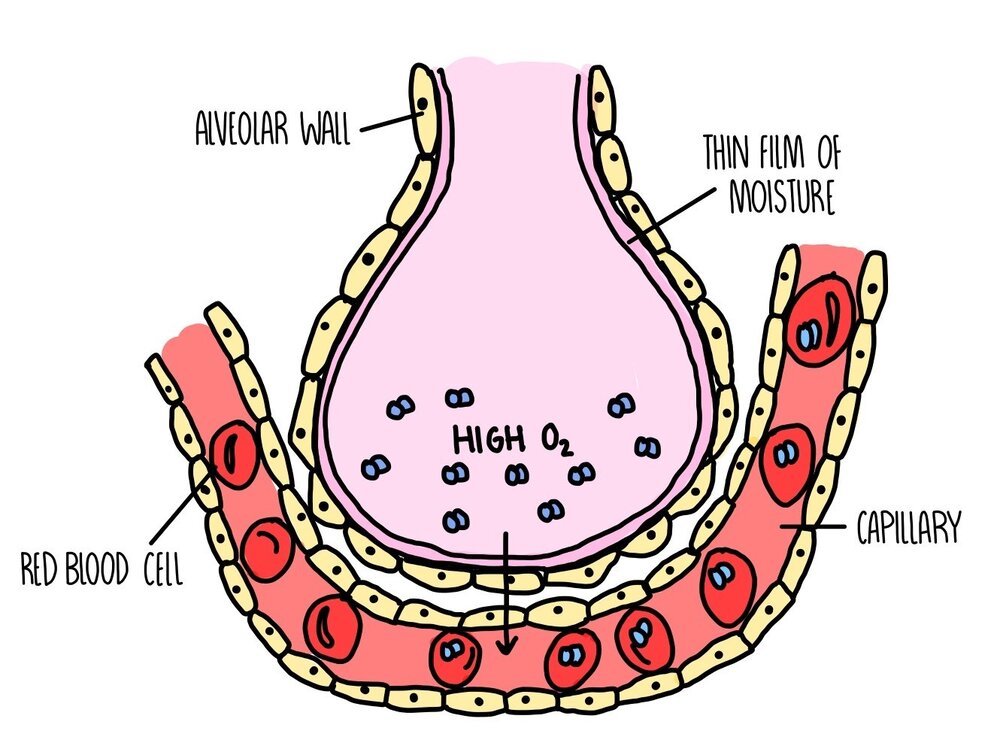
Adaptations of the lungs
Each alveolus is adapted to make gas exchange as efficient as possible:
Large surface area: there are approximately 700 million alveoli in our lungs with a combined surface area of 70 square meters.
Good blood supply: lots of capillaries surround each alveolus
Short diffusion distance: the walls of both the alveoli and capillaries are just one cell thick
Moist surfaces: the liquid on the surface of alveoli dissolves gases and facilitates diffusion
Inhalation and exhalation: breathing in and out replaces the air in the alveoli, maintaining a steep concentration gradient.
Location in the centre of the body: the lungs are right in the middle of our body, inside our thorax. The core of our body is the warmest, giving the gas molecules more heat energy and making them move around faster (so quicker diffusion).
Fick’s Law
Fick’s Law describes how the rate of diffusion is affected by the surface area, concentration gradient of gases and the thickness of the exchange surface. It is summarised in the equation below:
Since the rate of diffusion is in a proportional relationship to the three factors above, this means that if the rate of diffusion doubles if:
Surface area doubles
Concentration gradient doubles
Diffusion distance halves
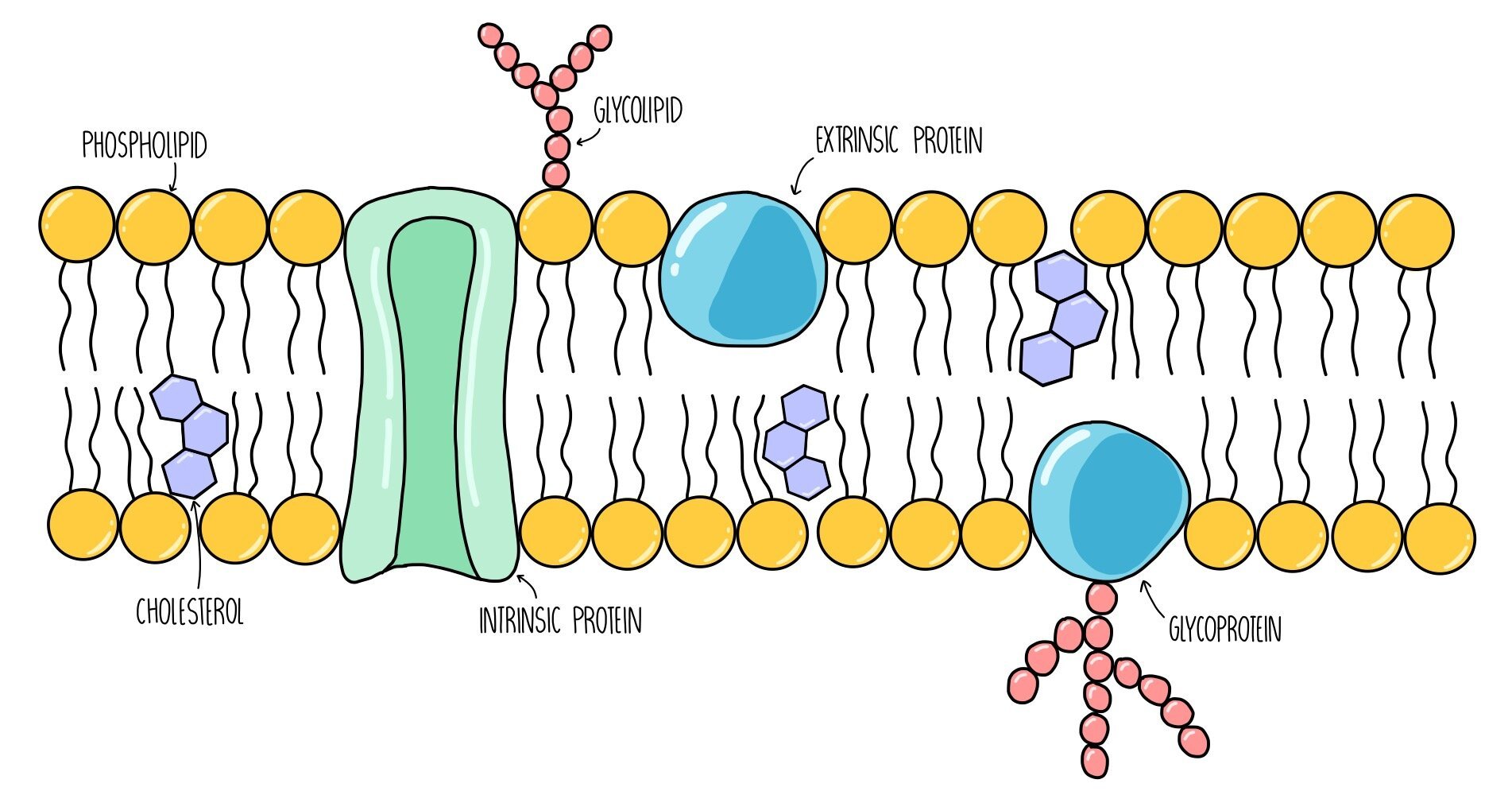
Fluid mosaic model
The structure of the plasma membrane is made up of a bilayer of phospholipids with proteins and cholesterol interspersed throughout the structure. The fluid mosaic model is used to describe the arrangement of molecules in the membrane - ‘fluid’ because the phospholipids are constantly moving around and ‘mosaic’ because protein molecules are scattered throughout the phospholipids like tiles in a mosaic.
We refer to this concept as a ‘model’ because it is the best representation of membrane structure based on the evidence which is currently available. As we learn more about the structure of the plasma membrane, the fluid mosaic model may be updated.
Components of the plasma membrane
Phospholipids: consist of a hydrophilic head group which faces the intracellular / extracellular fluid and two hydrophobic tails which point towards each other, away from water. They are the main component of the plasma membrane and form a barrier to anything which is not lipid-soluble (such as ions and glucose).
Glycoproteins: these are proteins with sugar molecules attached. They act as recognition sites and antigens - antigens are like little ‘flags’ on the surface of our cells which allows our body to detect which cells are our own and which cells are foreign.
Glycolipids: these are phospholipids with sugar molecules attached. They have a similar function to glycoproteins - they also act as recognition sites and antigens. They also increase membrane stability by forming hydrogen bonds with water molecules.
Cholesterol: cholesterol is a lipid which slots in between the phospholipid tails, pushing them closer together. It regulates the stability and fluidity of the plasma membrane.
Intrinsic proteins: these are proteins which span both bilayers of the plasma membrane. They act as channels or carrier proteins to transport water-soluble molecules.
Extrinsic proteins: these are proteins which are found on the surface of the plasma membrane. They usually function as enzymes and catalyse chemical reactions inside the cell.
Transport across cell membranes
Molecules can make their way across the plasma membrane in one of three ways: osmosis (if the molecule is water), diffusion (if it is a molecule moving down its concentration gradient) or active transport (if it is a molecule moving against its concentration gradient. For larger substances to get into or out of the cell, such as proteins or carbohydrates, they will rely on processes called endocytosis and exocytosis.
Osmosis: osmosis is the movement of water molecules down its concentration gradient across a partially permeable membrane. It is a passive process so does not require energy in the form of ATP. Osmosis is responsible for the movement of water molecules into the root hair cells of plants, for example.
Simple diffusion: this is the movement of molecules down their concentration gradients. When molecules move by simple diffusion, they pass directly through the phospholipid bilayer. It is a passive process which means that no energy is required. Oxygen and carbon dioxide move by simple diffusion when they pass from the alveoli into the bloodstream during gas exchange.
Facilitated diffusion: facilitated diffusion involves the movement of molecules down their concentration gradients. It differs from simple diffusion in the fact that a carrier protein or a channel protein within the cell membrane helps them get from one side to the other. This is also a passive process. An example of facilitated diffusion is the movement of glucose molecules into liver cells through glucose transporter proteins embedded in the plasma membrane.
ACTIVE TRANSPORT
Active transport: when molecules move against their concentration gradients (so from a region of low concentration to a region of high concentration), they do so by active transport. This involves a carrier protein which carries the molecule from one side of the membrane to the other. It is an active process and uses ATP to release energy. An example is the transport of glucose from the villi of the intestine into the bloodstream.
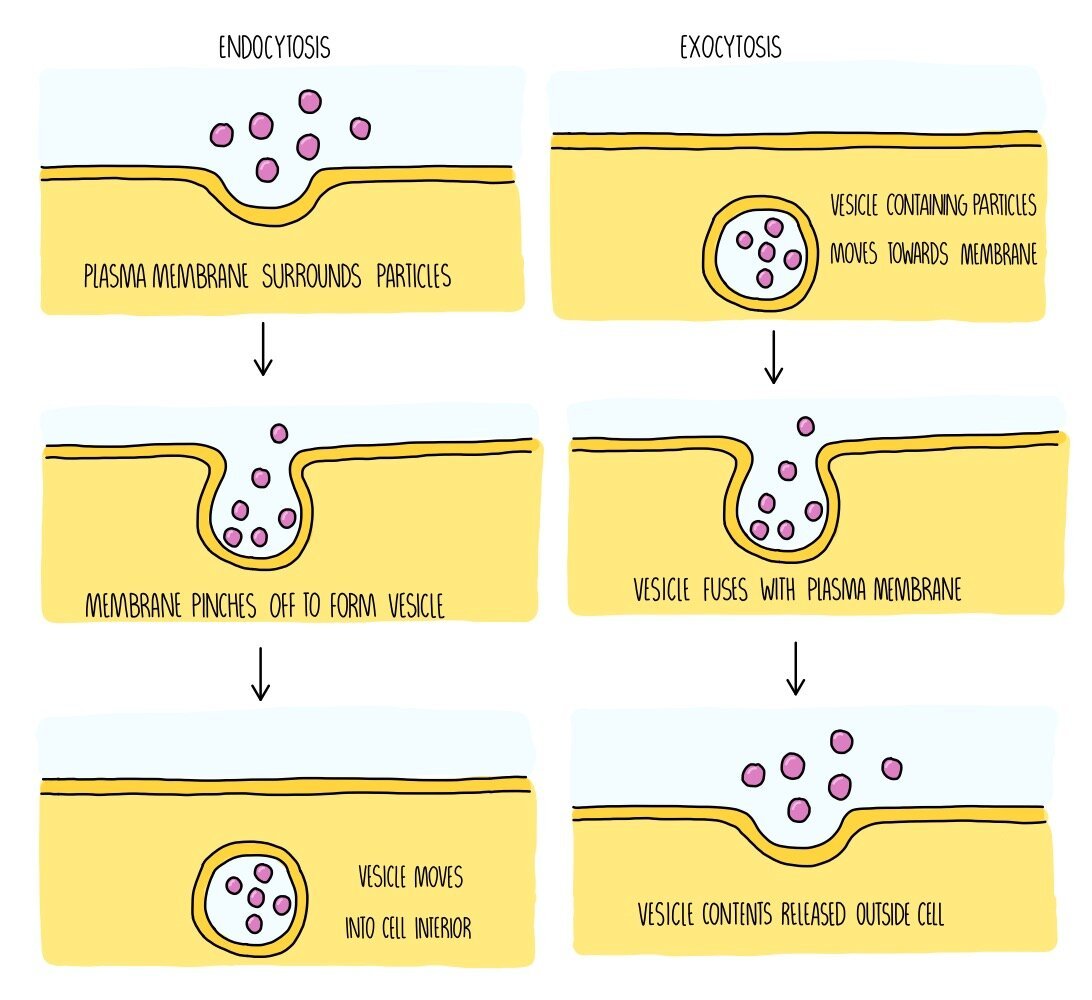
Endocytosis: if substances are too large to cross the membrane, they enter the cell by endocytosis. The cell surrounds the substance and folds its membrane around it. The membrane then pinches off to engulf the substance, which causes a vesicle to form inside the cell containing the ingested substance. This is an active process so will require energy in the form of ATP. An example of endocytosis is when phagocytes carry out phagocytosis, in which the phagocyte engulfs a whole bacterium in order to destroy it.
Exocytosis: when large substances need to leave the cell, such as hormones and digestive enzymes, they do so by exocytosis. These substances will be contained inside vesicles which move towards the plasma membrane and fuse with it. This causes the substances to either be released outside of the cell or they will be inserted straight into the membrane (for example, if the substance is a membrane protein). Exocytosis is an active process which requires ATP.
Investigating cell membrane structure
The permeability of cell membranes is affected by things like temperature, pH and ethanol. You may be asked to describe an experiment to determine the effect of one of these factors on membrane permeability. These experiments using involve plant cells which contain a coloured pigment, such as beetroot, since we can measure the amount of membrane permeability depending on how much pigment leaks out of the cells and into the surrounding solution. The method for this type of experiment is outlined below:
- Prepare eight cylinders of beetroot of equal size. Make these samples as similar as possible, e.g. by cutting from the same part of each plant. Rinse each piece to remove any pigment released during cutting.
- If you are investigating the effect of temperature, prepare eight water baths of varying temperatures ranging from 0 - 70oC.
- Prepare a series of test tubes containing the same volume of water (e.g. 10 cm3). Place the tubes in different water for five minutes.
- Place a single sample of beetroot into each of the eight test tubes. Leave for 15 minutes.
- Use forceps to remove the pieces of beetroot from each tube. Keep the coloured liquid and transfer into a cuvette.
- Use a colorimeter to measure how much light is absorbed by each liquid. The darker the solution (i.e. the more permeable the membrane), the more light is absorbed.
- Draw a graph plotting absorbance against temperature.
Temperature and membrane permeability
At temperatures below freezing, the permeability of cell membranes increases since the proteins in the membrane unfold and become deformed. The molecules in the membrane have low amounts of energy so cannot move around much. The phospholipids become closely packed together which makes the membrane rigid. When temperatures fall low enough for ice crystals to form, these can puncture the membrane which increases its permeability when the cell membrane re-thaws, damaging the cell.
Between temperatures of 0oC and 45oC, membranes are partially permeable. As temperature increases, the components in the membrane gain kinetic energy and move around more. The more fluid the membrane is, the more substances it allows through.
As temperatures exceed 45oC, permeability increases rapidly because proteins in the membrane become denatured and start to unravel. In addition, water inside the cell cytoplasm expands, putting pressure on the cell membrane and creating gaps within the bilayer.
Ethanol and membrane permeability
Ethanol is a non-polar solvent so it is able to dissolve non-polar substances such as lipids. This means that if you place a cell in ethanol, its membrane will become permeable and allow substances to leak into and out of the cell. As the ethanol concentration increases, membrane permeability will increase. If the ethanol concentration is high enough, enough phospholipids will dissolve to cause the plasma membrane to disintegrate completely which will kill the cell.