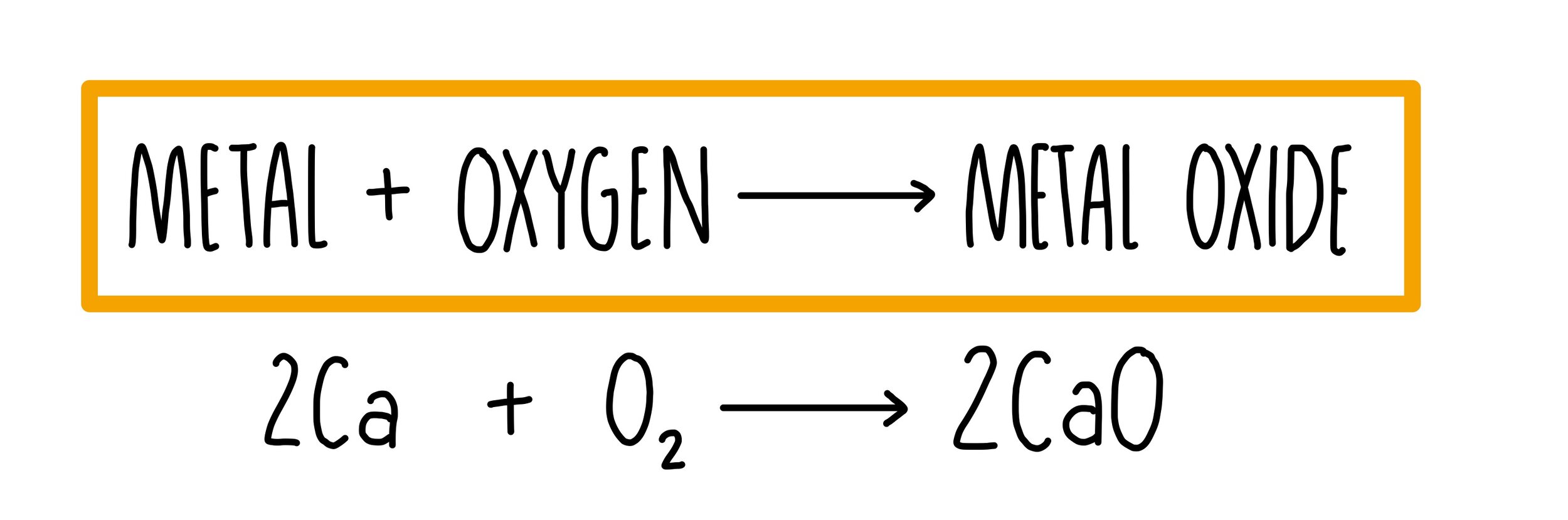Group 2
Redox reactions
- The elements in group 2 all have two electrons in their outer shells so form positive ions with a +2 charge.
- Group 2 is in the s block of the periodic table so their electron configurations will all end with s2.
- They are referred to as the alkaline earth metals since they react with water to form alkalis (metal hydroxides) and hydrogen.
- This is a redox reaction - the oxidation state of the group 2 element increases from 0 to +2, therefore it is oxidised. Hydrogen is reduced, since its oxidation number decreases from +1 to 0.
Group 2 metals react with oxygen to form metal oxides.
This is a combustion reaction (since oxygen is involved).
It’s also redox — the group 2 element is oxidised and oxygen is reduced.
Group 2 metals react with dilute acid to form a salt and hydrogen.
The group 2 metal is oxidised while hydrogen is reduced.
Reactivity
As you go down the group, the reactivity of the group 2 metals increases. This is because:
The outer electrons get further away from the nucleus, reducing the nuclear attraction.
Electron shielding increases, further reduces the attraction between the positive nucleus and the outer electrons.
So the outer two electrons are more easily lost to form +2 ions.
This also explains why ionisation energy decreases as you go down the group — it gets easier to remove an outer electron so less energy is needed.
Reactions of group 2 compounds
Group 2 oxides are bases, which neutralise acids. For example, calcium oxide neutralises hydrochloric acid to form a salt and water.
Group 2 oxides also react with water to form alkalis (metal hydroxides). Remember that an alkali is simply a soluble base, which releases hydroxide (OH-) ions in solution. For instance, calcium oxide reacts with water to form magnesium hydroxide. In solution, calcium hydroxide will dissociate into calcium ions (Ca2+) and hydroxide ions (OH-).
The exception is magnesium hydroxide, which is only partially soluble. It doesn’t release hydroxide ions quite as readily as the other group 2 hydroxides. As you go down group 2, the group 2 hydroxides get more and more soluble, which means that the solutions they form are increasingly alkaline.
Since group 2 compounds act as bases, they are often used to neutralise acids:
As antacids – magnesium hydroxide and calcium carbonate are used in indigestion tablets to neutralise excess stomach acid.
Calcium hydroxide (aka slaked lime) is used in farming to neutralise acidic soil.