Group 1 (Alkali Metals): Answers
What is an alkali and why are the group 1 elements referred to as the ‘alkali metals’?
An alkali is a substance which releases hydroxide ions when it dissolves. The group 1 elements are also called the ‘alkali metals’ because they all form metal hydroxides (alkalis) when they react with water. For example, lithium will react with water to produce lithium hydroxide.
How does the reactivity of the alkali metals change as you go down the group?
As you go down the group (from lithium to francium), the elements become more reactive.
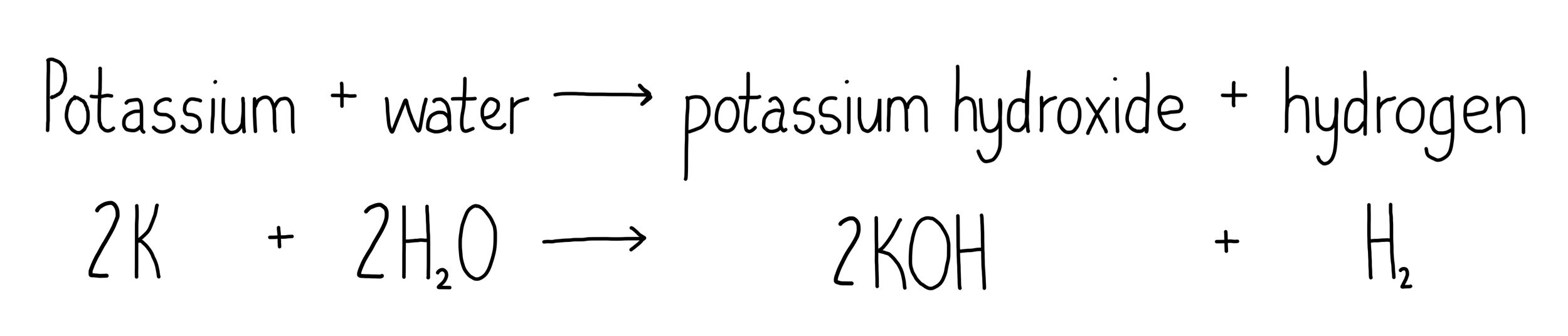
Write a word equation and a symbol equation for the reaction of potassium with water.
Describe the observations you would make if you added lithium, sodium and potassium to separate test tubes filled with water.
Placing lithium in water would cause it to fizz and slowly disappear. Sodium would fizz faster and disappear more quickly compared to lithium. Potassium will react the most violently of the three, burning with a purple flame and disappearing extremely quickly.
Write a word and symbol equation for the reaction of rubidium with oxygen.
Explain the trend in reactivity of the group 1 metals.
As you go down the group, the outer electron is further away from the positive nucleus. This means there is less attraction between the atom and its electron, so it can be more easily removed. That’s essentially whats happening when a group 1 element takes part in a chemical reaction - it’s getting rid of its single outer electron.