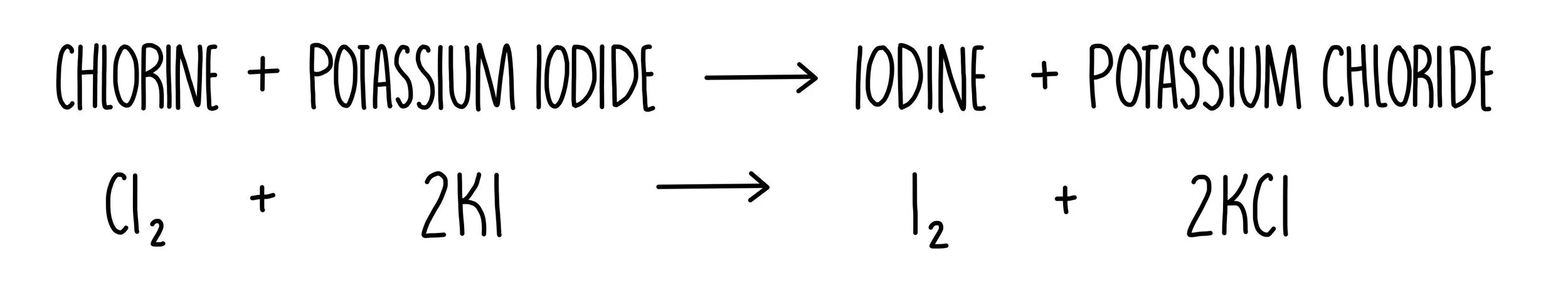Groups 0, 1 and 7
Elements in the same group all react in the same way. Here you’ll learn about the noble gases (group 0), alkali metals (group 1) and the halogens (group 7).
Group 0
The last group of the periodic table is group 0 (sometimes also called group 8) and is made up of the noble gases. These elements are all gaseous at room temperature and have a complete outer shell of eight electrons, except helium which only has two electrons. Since these elements have a complete outer shell they are unreactive so do not form molecules or compounds.
As you go down group 0, from helium through to radon, the boiling point increases. This is because the elements have more and more electrons as you go down the group, which means that they form stronger intermolecular forces. These stronger intermolecular forces need more energy to overcome when the element is being converted from a liquid to a gas.
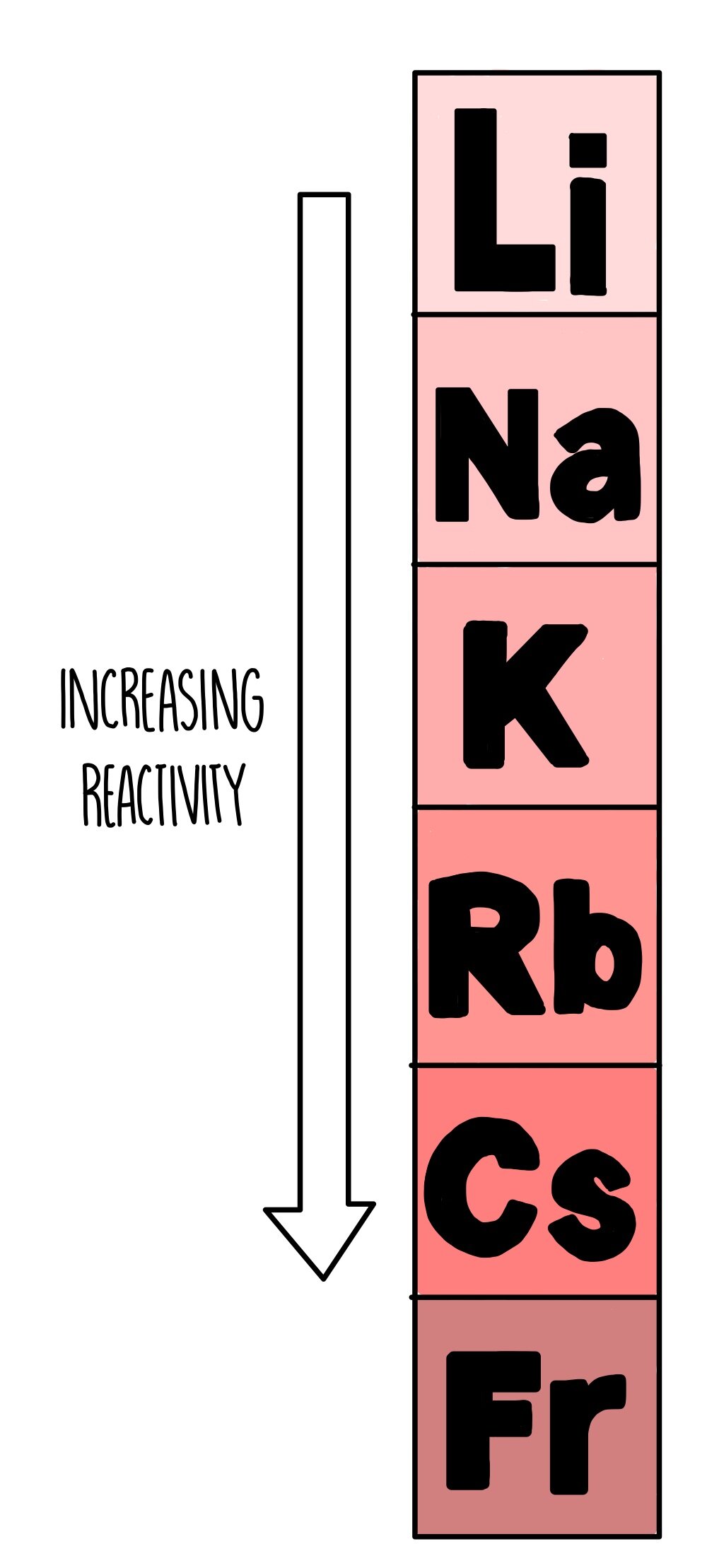
Group 1
Lithium, sodium and potassium all react vigorously with water to produce a metal hydroxide and hydrogen. The metal hydroxide is an alkali which simply a substance which release hydroxide ions (OH-) into solution. Because all of the group 1 metals react with water to form alkalis, they are given the name 'alkali metals'. The equation for the reaction of lithium with water is written below:
As you go down group 1, the reactivity of the metals increases. Lithium and sodium themselves are pretty volatile, but once you reach potassium with its fiery temper and serious anger management issues, you’re in for an explosion. The elements below potassium are even more reactive, but they aren’t nearly as common and only found in small amounts.
If you react each of the first three group 1 metals with water you should make the following observations:
Lithium fizzes slowly until it disappears
Sodium fizzes rapidly and disappears faster
Potassium burns violently with a purple flame and disappears extremely quickly
The group 1 metals also react with oxygen in the air to form a metal oxide. The equation for the reaction with sodium is written below:
As before, the elements further down the group will react with oxygen more quickly. The group 1 metals are usually kept in oil to prevent this reaction from happening. Click here to watch a video of scientists running from potassium explosions.
Reason for the trend in reactivity
When the group 1 metals react, they form positive ions with a +1 charge by losing their single outer electron. As you go down the group, the number of electron shells increases so the electron that needs to be lost is further away from the nucleus. The greater the distance between the electron and the nucleus, the weaker the attraction between the nucleus and the electron. Since the nucleus doesn’t have such a tight grip on this outer electron, it is more easily taken by another atom to form a positive ion.
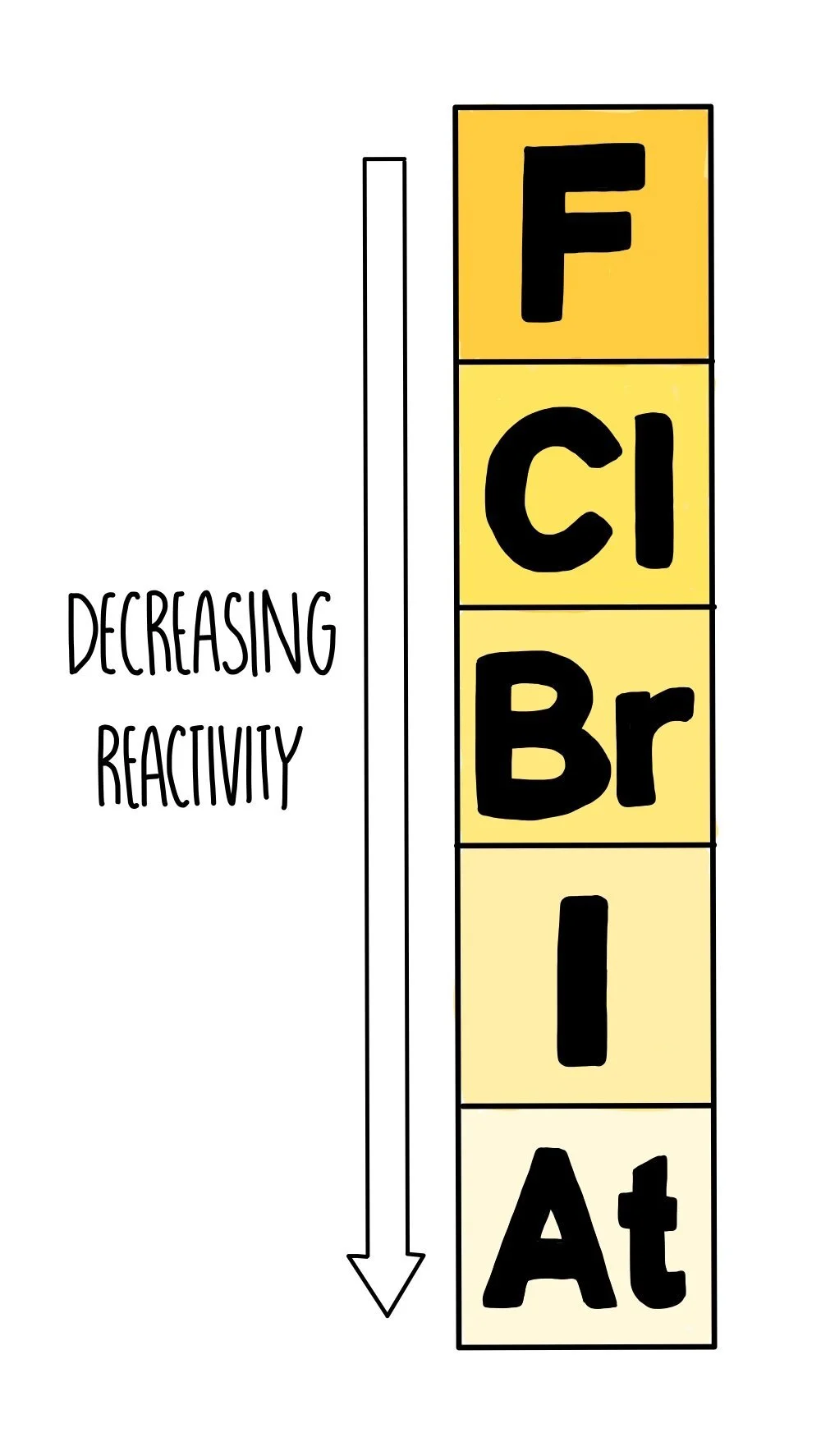
Group 7
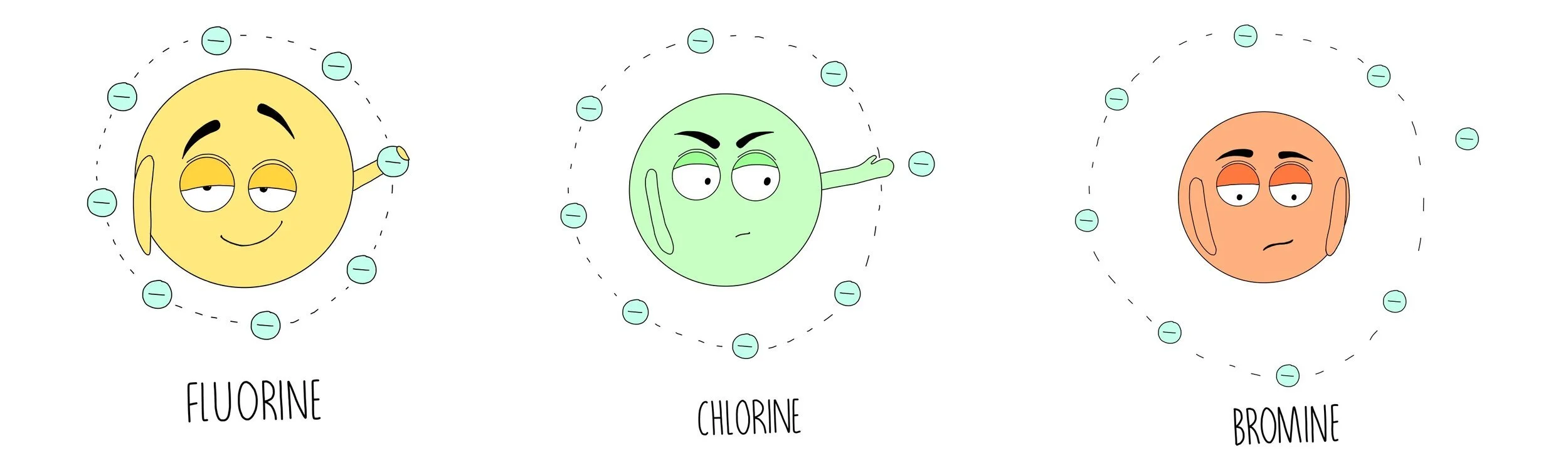
The halogens are the elements found in the second-to-last group of the Periodic Table. They all have seven electrons in their outer shell and since they need only one more to complete their octet they are fairly reactive. The halogens typically form ionic compounds with elements in group 1 which have one outer electron that the group 7 elements can’t resist taking.
As you go down the halogens, from fluorine to astatine, the elements become darker in colour and have a higher boiling point. Boiling point increases as you go down the group because the mass of each element increases and they have more electrons around their nuclei. The more electrons an element has, the more intermolecular forces (London dispersion forces) it can form. Each halogen has the following characteristics at room temperature:
Fluorine is a pale yellow gas
Chlorine is a poisonous green gas
Bromine is a toxic red-brown liquid
Iodine is a dark grey solid which gives off a purple vapour when heated
Astatine is a black solid
Unlike the group 1 metals, reactivity decreases as you go down the halogens. This means that fluorine, at the top of the group, is the most reactive. Fluorine is so eager to react with anything that it is almost never found as a pure element and it is so dangerous to work with that scientists avoid handling it in reactivity experiments. Chlorine is less reactive and much more manageable, and added to water in small quantities to kill microorganisms to make it safe to drink.
The group 7 elements want to gain one more electron so that they have a stable electronic structure. The smaller the atom, the easier it is to grab an electron from another atom, making the atom more reactive. As you go down group 7, the atomic radius increases and it becomes more difficult to attract another electron.
In displacement reactions, a more reactive element will displace (replace) a less reactive one. Think of it as the introduction of an attractive geordie on love island, forcing someone else out of a relationship and leaving them by themselves. Displacement reactions involving halogens and halogen ions (halides) can be used to provide evidence for the order of reactivity of the halogens.
An example is the addition of chlorine to a solution of potassium iodide. Chlorine is more reactive than iodine so will take its place in the molecule, resulting in the formation of potassium chloride and iodine. The equation for the reaction would look like this:
Let’s say we add iodine to a solution of potassium bromide. This time we’re adding an element which is less reactive than the halogen in the compound so no reaction will take place.