Haloalkanes
Properties of haloalkanes
Haloalkanes are alkanes where at least one hydrogen atom is replaced with a halogen atom. For haloalkanes with two different halogen atoms attached, these are named in alphabetical order.
The electronegative halogen atom creates a polar C—X bond (X = halogen). The slightly positive carbon means that haloalkanes can be attacked by nucleophiles – these are things with a lone pair of electrons, such as OH-, CN- and NH3. Water is also a nucleophile, as it has two lone pairs, but it reacts more slowly than other nucleophiles.
Substitution reactions
Haloalkanes are hydrolysed into alcohols in nucleophilic substitution reactions. The reaction requires warm aqueous alkali, such as sodium hydroxide or potassium hydroxide. The reaction mechanism involves the following steps:
A hydroxide ion nucleophile attacks the slightly positive carbon of the carbon-halogen bond.
The electrons in the C—X bond jump onto the halogen, causing the covalent bond to break heterolytically. Both bonding electrons are taken by the halogen.
An alcohol and a halide ion are formed.
The same reaction can take place with water as the nucleophile – the difference is that the reaction is much slower. As well as the alcohol and halide ion, a hydrogen ion is also formed.
Rate of hydrolysis
Different haloalkanes will hydrolyse at different rates (their bonds will take different amounts of time to break). This is due to differences in the strength, or bond enthalpy, of different carbon-halogen bonds.
The C—F bond has the highest bond enthalpy, so it requires the most energy to break. As you go down the group, enthalpy decreases and it gets easier to break the C—X bond. This means that fluoroalkanes react the slowest and iodoalkanes react the fastest.
This can be tested experimentally using the following method:
Set up 3 test tubes containing a different haloalkane dissolved in ethanol.
Convert the haloalkanes into alcohols by reacting with warm aqueous alkali or water. This is the nucleophilic substitution reaction described above.
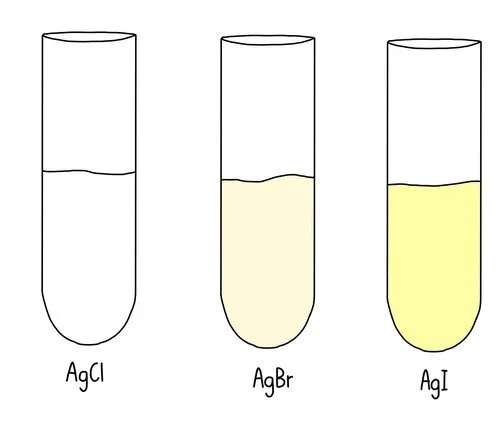
Add silver nitrate – this will react with the halide ions formed in the substitution reaction. Silver halide precipitates are formed.
Silver chloride is a white precipitate which is soluble in dilute ammonia solution
Silver bromide is a cream precipitate which is soluble in only concentrated ammonia solution
Silver iodide is a yellow precipitate which is insoluble in aqueous ammonia
Time how long it takes for each precipitate to form. We should expect to see a pale yellow solid (AgI) appearing first, followed by the cream solid (AgBr) then the white solid (AgCl).
Impact of haloalkanes on the environment
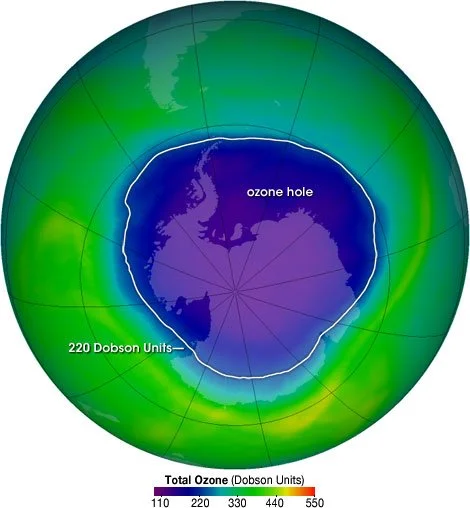
A map showing the size of the ozone hole over Antartica in 2004. Credit: NASA Ozone Watch
Chlorofluorocarbons (CFCs) are haloalkanes containing chlorine and fluorine and are very stable due to their strong bonds. They used to be a component of things like aerosols and fridges before they were banned when scientists realised that they were producing holes in the ozone layer.
The ozone layer is a layer in the upper atmosphere which is able to absorb much of the UV radiation from the sun, protecting us from sunburn and skin cancer. It is made up of ozone, molecules in which three atoms of oxygen are bonded together (O3).
It's formed when UV radiation causes an oxygen molecule to break by homolytic fission, forming two oxygen free radicals. Free radicals are species with unpaired electrons and are extremely reactive. The radicals react with oxygen molecules to form ozone.
Chlorine radicals can react with ozone, catalysing its breakdown into oxygen. The chlorine radicals are formed when the C—Cl bonds in chlorofluorocarbons are broken by UV radiation.
Nitrogen oxide radicals catalyse the same reaction and also contribute to holes in the ozone layer. Nitrogen oxides are produced by vehicle engines and thunderstorms. In the atmosphere, UV light can break one of the N—O bonds to form NO radicals. These react with ozone in the exact same way as chlorine radicals.
Notice that in both cases, the Cl and NO free radicals are acting as catalysts, because they are regenerated and are unchanged by the end of the reaction.
CFCs were banned in the 1970s. Since then, scientists have been working on alternatives:
Hydrofluorocarbons (HFCs) – these are also broken down by UV light but they don’t contain chlorine so are less damaging to the ozone layer. However, they are greenhouse gases and contribute to global warming.
Hydrocarbons are sometimes used to replace CFCs in fridges – these are also greenhouse gases.
Pump spray systems and nitrogen propellants are now used in aerosols. Ammonia gas is often used in industrial fridges and freezers. Both are much better for the environment than CFCs.
Because of these changes, the hole in the ozone layer is gradually shrinking and is the smallest it has been in decades.