Mixtures and Chromatography
A mixture is a substance made up of different elements or compounds which are not chemically bonded together. Mixtures can be separated into their components by chromatography.
Pure substances
A substance is pure if it consists only of a single element or compound and has not been mixed with any other substances or had anything added to it. A substance that contains other substances (impurities) is impure. Pure substances differ from impure substances in their melting and boiling points – pure elements and compounds will melt and boil at a very specific temperature whereas impure substances will melt or boil over a range of temperature.
Formulations
Paint is a formulation, consisting of pigment, solvent and binder.
A formulation is a mixture of substances that has been designed as a useful product. Each chemical in the mixture will have a particular purpose. The chemicals will be added in carefully measured quantities to ensure that the product has the correct properties.
For example, paint is a formulation which contains pigment, solvents and binder. The pigment gives the paint its colour, the solvent dissolves the pigment to form a liquid and the binder holds the pigment in place. It is important that these three components are combined in the correct quantities to ensure that the paint has the right properties.
Other examples of formulations include:
Medicines
Fuels
Cleaning products
Food
Alloys
Fertilisers
Chromatography
Paper chromatography is used to separate a mixture of soluble substances, such as separating dyes within food colouring or plant pigments. It involves a stationary phase (the part of the equipment which doesn’t move) which is a sheet of paper and a mobile phase (the thing which moves) which is the solvent.
A line is drawn near to the bottom of the piece of paper - this is the starting line where the dyes will be placed. It is important that the line is drawn in pencil because the ink from pens will also dissolve in the solvent and cause a mess.
The paper is placed in a tank containing some solvent and a lid is placed on top of the tank to prevent evaporation of the solvent. The solvent need to be below the starting line so that the dyes do not dissolve in the solvent before they can move along the paper.
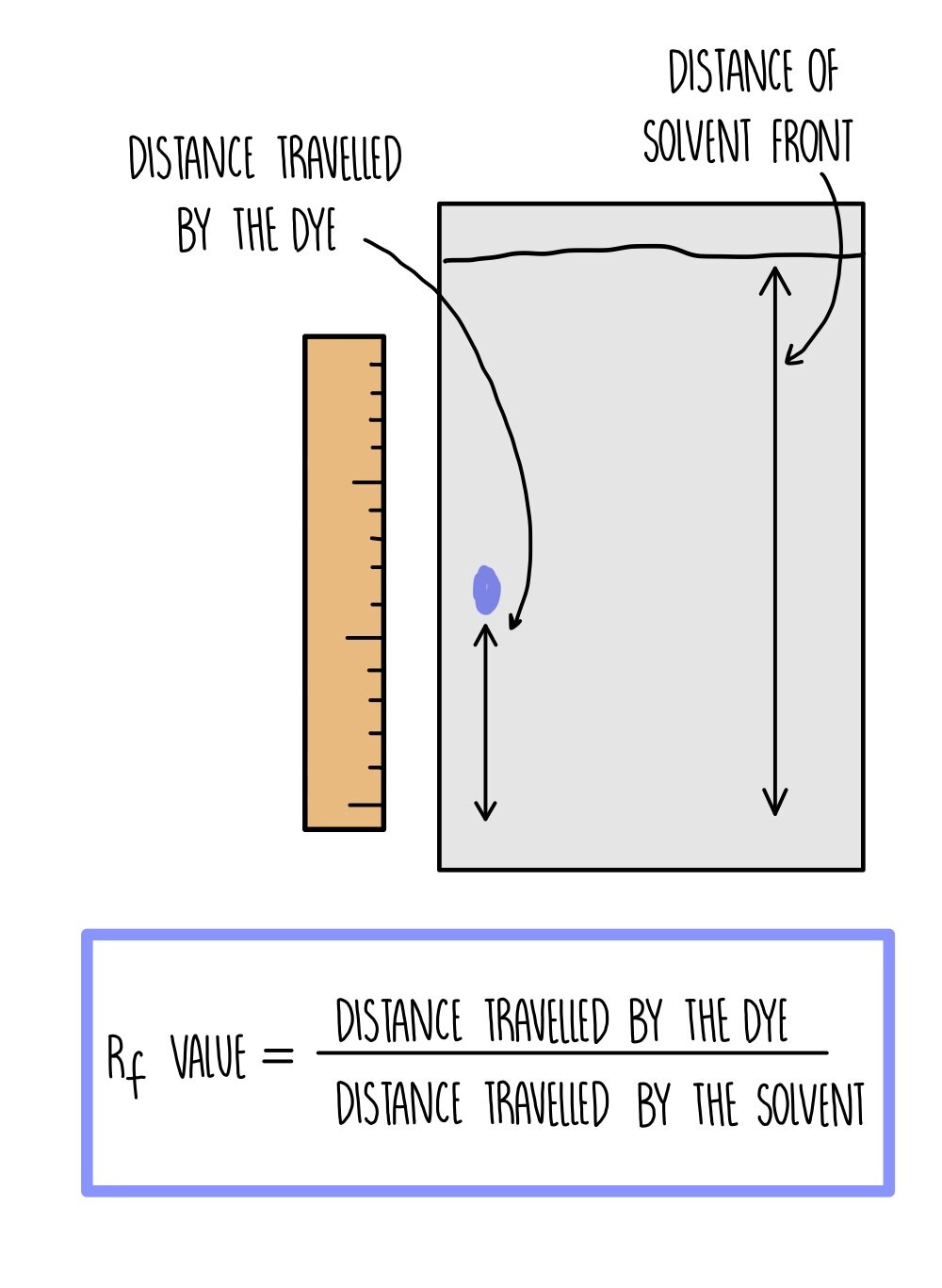
As the paper absorbs the solvent, the solvent moves further up the stationary phase. The dyes are carried up the paper along with the solvent with different dyes moving up the paper to different extents. The total distance travelled by the solvent is the solvent front.
An Rf value is calculated for each dye using the equation: Rf = distance travelled by dye / distance travelled by solvent
Because different compounds have different Rf values, we can use chromatography to identify the compounds present by comparing their Rf value to the published values in a data book. We can also use chromatography to determine whether a sample is made up of a pure compound or a mixture since a pure compound will produce a single spot whereas a mixture will separate into several spots on the chromatography paper.
Did you know…
A type of chromatography called gas chromatography can be combined with other methods of chemical analysis to identify substances. It is used in breathalysers to identify alcohol in the breath of drunk drivers and has even been used to test for COVID-19.
Next Page: Identifying Gases