Muscle Contraction
Skeletal muscle
There are different types of muscle - for example, skeletal muscle, smooth muscle and cardiac muscle. Skeletal muscle is the type of muscle used for physical movement such as when we pick up objects or go for a run. Skeletal muscle is attached to bone through tendons and it contracts or relaxes in order to move the bone that it is connected to. Muscles can work in antagonistic pairs so that when one muscle contracts, the other relaxes.
The movement of your arm at the elbow joint involves two muscles - your biceps and your triceps. When your bicep contracts, your triceps relaxes. This pulls the bone so that your arm bends at the elbow. Biceps are referred to as flexors because they cause the bone to bend (flex) when they contract. On the other hand, the relaxation of the biceps and contraction of the triceps causes the extension (straightening) of the arm. Triceps are referred to as extensors because they cause the bond to extend when they contract.
Structure of skeletal muscle
Skeletal muscle is made up of bundles of long muscle cells, called muscle fibres. The organelles inside muscle cells tend to have the prefix sarco- stuck to the front of their name. The cell membrane of muscle cells is called the sarcolemma and the cytoplasm of muscle cells is called the sarcoplasm. The sarcolemma folds into the sarcoplasm, creating something called transverse (T) tubules which help to spread electrical impulses throughout the cell. Muscle cells have a special organelle called the sarcoplasmic reticulum, which stores calcium ions for muscle contraction. Muscle cells also differ from other cells in that they contain many nuclei (they are multinucleate) and lots of mitochondria to generate ATP for muscle contraction. In addition, muscle fibres contain long cylinders of protein called myofibrils, which enable the muscle fibre to contract.
Sarcomeres
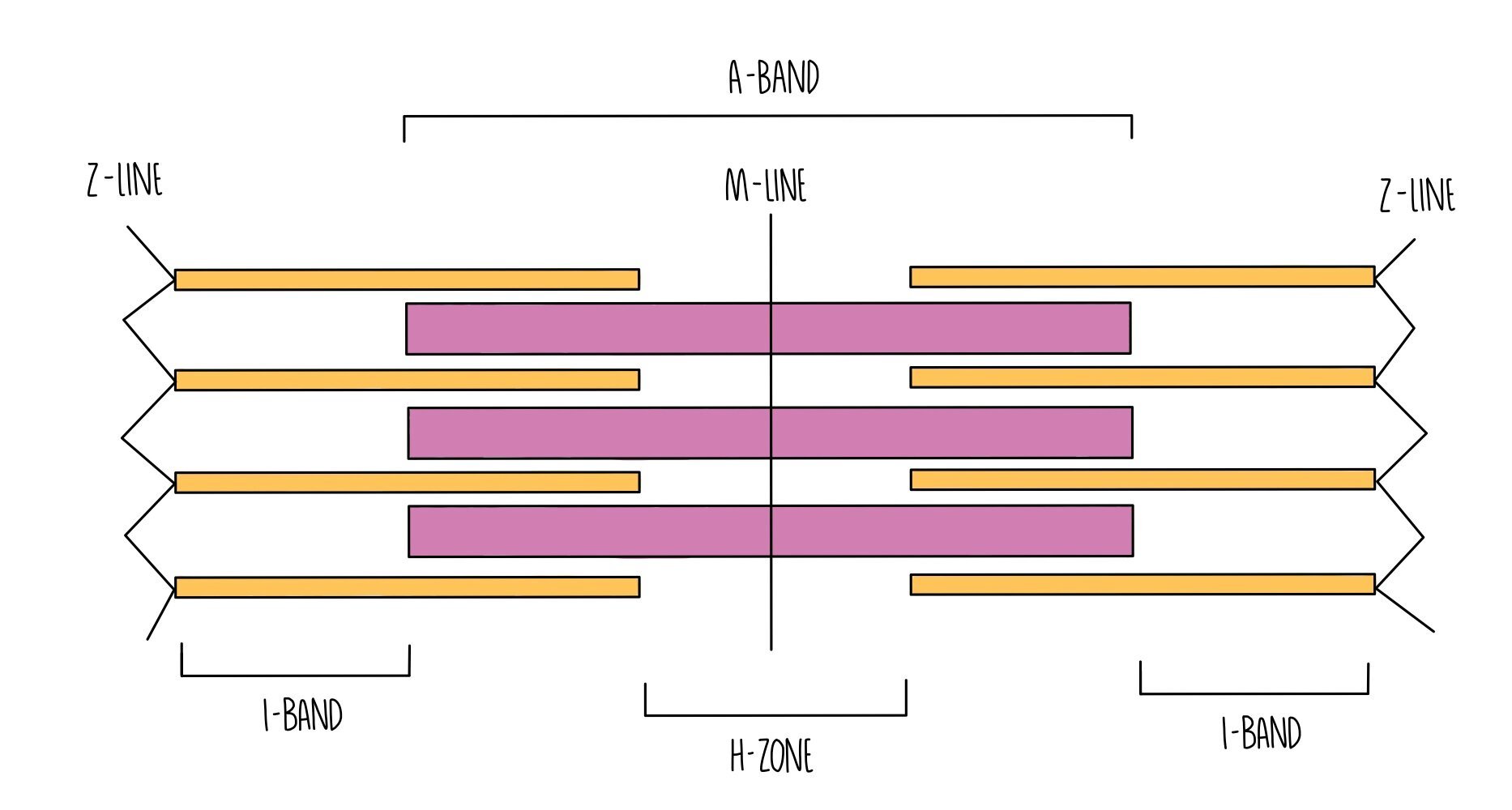
Myofibrils are made up of many short units called sarcomeres, which are made up of two types of myofilament: myosin and actin.
Myosin is a thick myofilament and appears as a dark band (called the A band) under the microscope.
Actin is a thin myofilament and appears as a light band (called the I band) under the microscope.
At the end of each sarcomere is a Z-line. Sarcomeres are joined together lengthways at the Z-line.
Right in the middle of the sarcomere is a region called the M-line.
The H-zone refers to the portion of the A-band which only contains myosin filaments (and not the portions where actin overlaps with myosin).
Sliding filament theory
When muscle fibres contract, the myosin and actin myofilaments move closer together by sliding over one another. This makes the sarcomere shorter and they contract. Remember that the actin and myosin myofilaments themselves don’t contract - they always stay the same length. As the muscle fibre relaxes, the myofilaments slide away from each other and move further apart, lengthening the sarcomere.
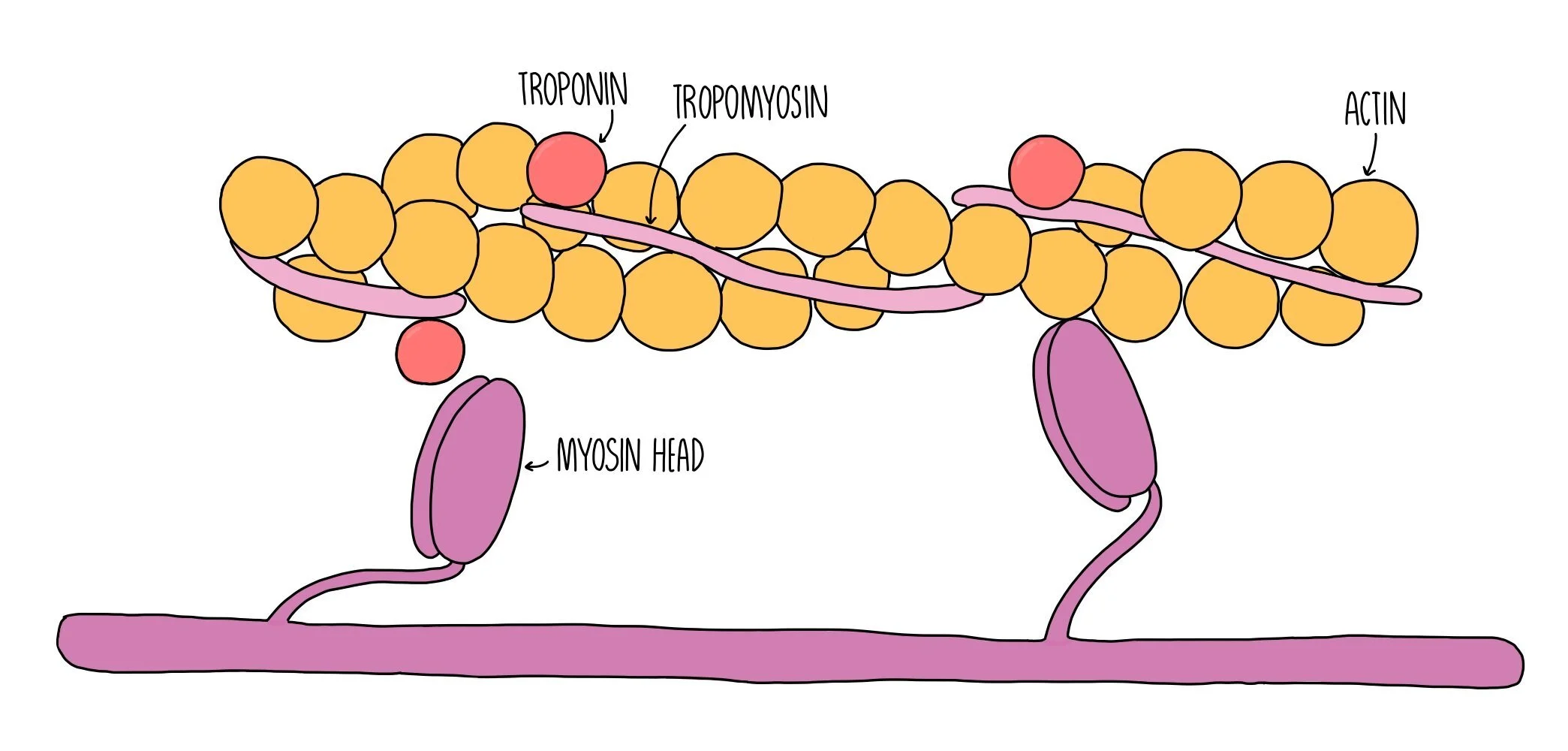
Myosin myofilaments possess head groups which contain binding sites for actin and ATP. Myosin heads are a globular and hinged, allowing them to move back and forth and enabling it to slide the actin filaments closer towards it. The region on actin where myosin binds is called the actin-myosin binding site. This binding site is blocked under resting conditions by two proteins called tropomyosin and troponin. When the muscle is not contracting, tropomysin covers the actin-myosin binding site and is held in place by troponin.
When an action potential (nerve impulse) arrives at a muscle fibre, a wave of depolarisation passes along the sarcolemma and down the T-tubules. This stimulates the sarcoplasmic reticulum to release calcium ions, which bind to troponin. Binding of calcium ions to troponin cause it to change shape, which pulls the tropomyosin out of the actin-myosin binding site. Now that the binding site is uncovered, the myosin head can bind to actin forming a bond called an actin-myosin cross bridge.
The release of calcium ions also activates the enzyme ATPase which catalyses the hydrolysis of ATP into ADP and inorganic phosphate. The energy released from ATP hydrolysis is used by the myosin head group to move backwards, pulling the actin filament closer towards it in a sort of rowing action which is referred to as a power-stroke. ATP hydrolysis also provides the energy to break the actin-myosin cross bridge. The myosin head can then reattach to a binding site further along the actin filament. The process is repeated, pulling the actin further and further towards the myosin filament. This shortens the sarcomere and results in muscle contraction.
When the muscle stops being stimulated, calcium ions move back into the sarcoplasmic reticulum by active transport, in a process which also requires ATP. The troponin molecules reform their original shape, pushing tropomyosin back into the actin-myosin binding site. Myosin can no longer bind to actin and the actin myofilaments slide back to their original position. The sarcomere lengthens and the muscle becomes relaxed.
Slow twitch and fast twitch skeletal muscle
There are two types of skeletal muscle fibres - slow twitch and fast twitch. Like their names suggest, slow twitch fibres contract slowly whereas fast-twitch muscle fibres can contract much quicker. Different muscles will have different proportions of slow and fast twitch fibres depending on their role. Muscles which are used for posture (such as the muscles in the back) have a higher proportion of slow-twitch fibres whereas muscles involved in rapid movement (such as those in the legs and the eyes) have a higher proportion of fast-twitch fibres. Slow twitch fibres can contract for long periods without getting tired and get their energy from aerobic respiration. These are the types of muscle fibres which are involved in longer, endurance-based sports. Fast twitch fibres fatigue easily and get their energy from anaerobic respiration. They are mostly used for short bursts of speed (e.g. a sprint). Slow twitch fibres have a reddish appearance due to the presence of high amounts of myoglobin - a red-coloured protein which stores oxygen. In contrast, fast-twitch fibres appear white because they have lower levels of myoglobin.