Organic Synthesis
Reflux and distillation
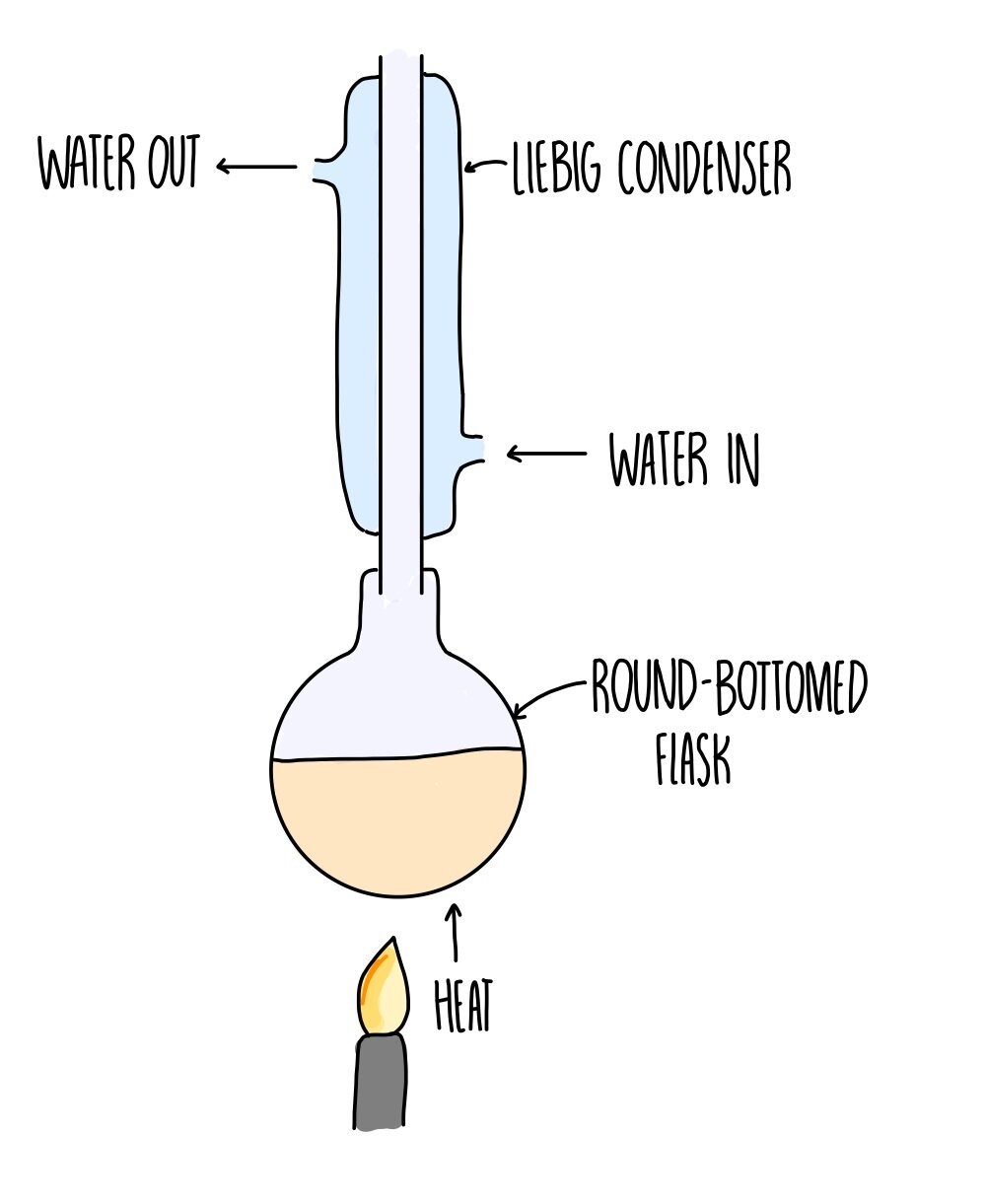
Refluxing a substance involves a repeated cycle of evaporation and condensation. Organic compounds often evaporate before they have had a chance to react, due to their high volatility (they evaporate easily). Condensing the compound returns it back to the flask so that it can react.
You need to use a round-bottomed flask fitted with a vertical Liebig condenser.
Reflux is used to oxidise primary alcohols into carboxylic acids. Partial oxidation produces aldehydes, so we need it to return to the flask to be completely oxidised.
Distillation involves evaporating the product of a reaction mixture and immediately removing it. You will still have your reaction mixture in the round-bottomed flask but this is attached to a condenser coming out to the side.
If you know the boiling point of the pure product, you can use a thermometer sticking into the flask to determine when it is condensing. If the product has a lower boiling temperature than the reactants, distillation can be used as a way of separating off the product as it forms, as it will evaporate and condense before anything else in the reaction mixture.
Distillation is used to oxidise primary alcohols into aldehydes. If this were to remain in the flask, it will fully oxidise to form a carboxylic acid. Distillation allows us to remove it straight away before it has a chance to be further oxidised. It can also be used to separate a mixture of liquids with different boiling points.
Separation
Separation is a technique that allows us to remove any impurities from an organic product, so long as the impurities are soluble in water. Once a reaction has finished, the impure product is poured into a separating funnel and water is added. You need to shake the funnel then allow it to settle into two layers – an aqueous layer (with the dissolved soluble impurities) and the organic layer (containing the pure organic product).
These layers won’t mix, since the organic layer is non-polar (hydrophobic) while the aqueous layer is polar (hydrophilic). You then remove the aqueous layer by opening the tap and letting it run out. Once the aqueous layer is removed, you can collect the organic layer in a beaker.
Because the organic product contains trace amounts of water, you might add an anhydrous salt to remove the remaining water. Salts such as magnesium sulfate or calcium sulfate are often used, forming hydrated sulfate salts when they bind to water. The salt is removed by filtration.
Reactions of functional groups
You can predict how a compound reacts based on its functional group:
Alkanes contain the C-C functional group and take part in free radical substitution reactions.
Alkenes contain the C=C functional group and take part in electrophilic addition reactions.
Alcohols contain the C-OH functional group and take part in nucleophilic substitution, oxidation, esterification and dehydration reactions.
Haloalkenes contain the C-X functional group and take part in nucleophilic substitution reactions.
Aldehydes contain the –CHO functional group and take part in oxidation reactions.
Carboxylic acids contain the –COOH functional group and take part in esterification reactions.
Synthetic routes
You also need to be able to describe how to convert one compound into another, which are summarised in the image below. All of these are described in more detail on previous chemistry pages – go back and look at these if any of these pathways look a bit unfamiliar.