Percentage Yield and Atom Economy
Percentage yield tells us how much of our reactants have been successfully converted into products, while atom economy tells us how many atoms in the reactants have been converted into our desired product. Both are important factors to consider if you are manufacturing a product using a chemical reaction.
Percentage Yield
The law of conservation states that no atoms are gained or lost in a chemical reaction. In theory, this means that if you start with 10 grams of reactants, you should end up with 10 grams of product. In reality, this is rarely the case for the following reasons:
The reaction might be reversible and won’t go to completion
Some product may be lost when it is separated from the reaction mixture
Some of the reactants may react in a different way to the expected reaction (e.g. if you add a metal with an acid, some of the metal may react with the oxygen in the air before it has chance to react with the acid)
The amount of reactant that is successfully converted into product is known as the yield. The more reactant converted into product, the higher the yield. When we compare this with the maximum amount that can be theoretically obtained, we call this the percentage yield. Percentage yield can be calculated using the equation:
Worked example
In a school laboratory experiment, a teacher combines sulfuric acid with magnesium to produce 6.25 g of magnesium sulfate. If the maximum yield is 8.5 g, calculate the percentage yield in this experiment.
Percentage yield = (actual yield / theoretical yield) x 100
Percentage yield = (6.25 / 8.5) x 100
Percentage yield = 73.5 %
Atom Economy
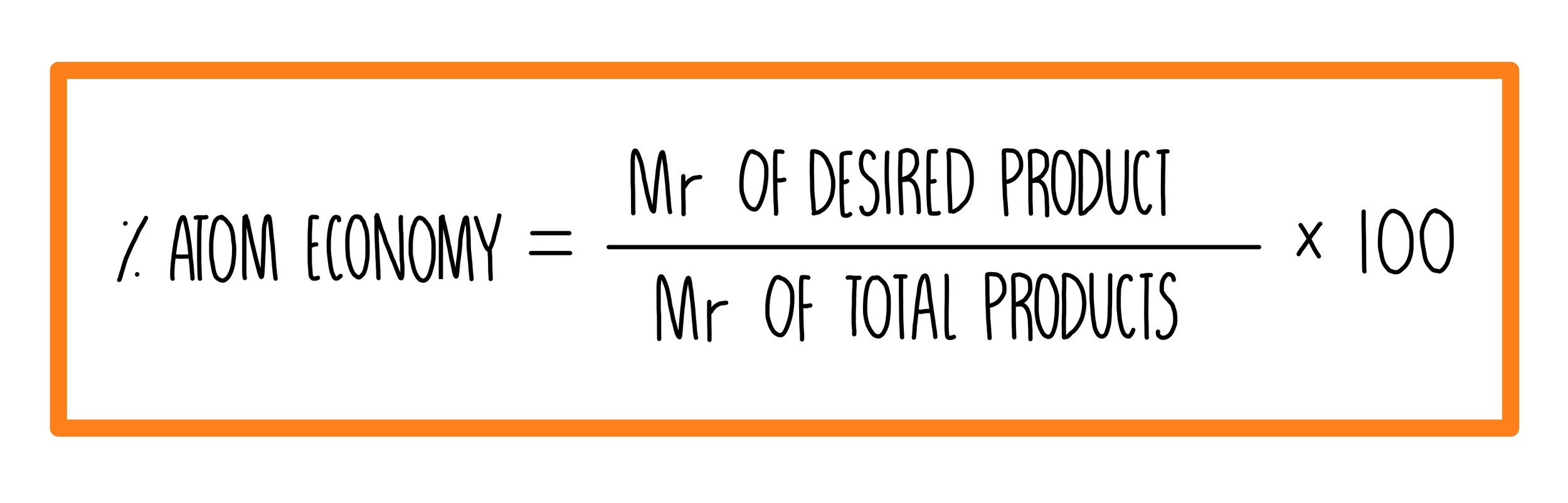
The atom economy of a reaction is a measure of the amount of reactants which are converted into useful products. The fewer waste products there are, the higher the atom economy. It is cheaper and more sustainable to use reactions with a higher atom economy. The percentage atom economy of a reaction is calculated using the formula:
Worked example:
Ethanol can be manufactured by fermentation of glucose in the presence of yeast. It can also be made through the hydration of ethene. Equations for both reactions are given below:
Calculate the atom economy for each of these reactions.
For the fermentation of glucose:
Mr of desired product (ethanol) = 92
Mr of total products = 92 + 88 = 180
(Mr of desired product / Mr of total product) x 100 = 51%
For the hydration of ethene, ethanol is the only product formed which means that the atom economy is 100% (i.e. all of the reactants are converted into useful product).
Did you know…
See that tiny dot in the middle of the two electrodes in the photo? That’s a single atom of strontium, trapped between two electrodes and suspended in an electric field. It’s the first time somebody has taken a photo of an atom that is visible to the naked eye, using an ordinary digital camera. Image credit: David Nadlinger - University of Oxford
Next Page: Concentration and Moles