Reactivity of Metals
When metals react they form positive ions by losing their outer electrons. The more readily a metal loses electrons, the more reactive it is. The reactivity series shows the order of reactivity of metals, which we can use to predict how they will be extracted from their ores.
Metal oxides
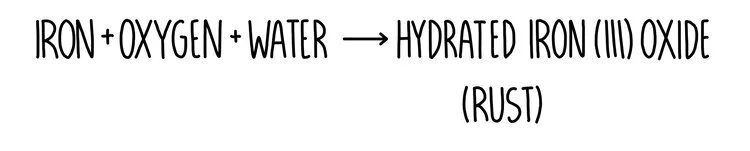
When a metal reacts with oxygen, a metal oxide is formed. For example, iron reacts with oxygen (and water) to form iron oxide (aka rust).
This is an example of an oxidation reaction because iron has gained oxygen. Remember that oxidation / reduction can be described in terms of electrons but it can also be described as a gain or loss of oxygen. A substance which has gained oxygen has been oxidised and a substance which has lost oxygen has been reduced.
The reactivity series
When metals react, they lose electrons to form positive ions. For example, when sodium (a metal) reacts with chlorine (a non-metal) to form the salt sodium chloride, the sodium has lost electrons to form positive sodium ions (Na+). The reactivity of a metal describes its tendency to form positive ions. The more reactive the metal, the more easily it forms positive ions.
The reactivity series lists the metals in order of reactivity, from the most reactive at the top (potassium) to the least reactive at the bottom (gold). You will need to learn this list for the GCSE exam and it’s much easier if you use a mnemonic to help you. For example, Phillip Schofield loves calling me a zombie - I can’t stand (the) guy.
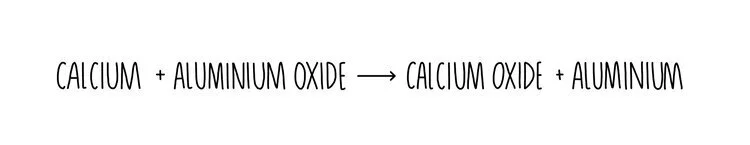
A more reactive metal will replace a less reactive one from a compound. For example, let’s say we add calcium to aluminium oxide. Calcium is higher up the reactivity series compared to aluminium so it will displace aluminium, forming calcium oxide. This happens because calcium binds more strongly to the oxygen.
Displacement reactions like the one shown above are redox reactions where one element is oxidised (loses electrons or gains oxygen) and the other is reduced (gains electrons or loses oxygen). Remember OILRIG: oxidation is loss and reduction is gain (of electrons). In our example above, we can see that calcium is oxidised since it gains oxygen and the aluminium is reduced.
Another example of a displacement reaction is the reaction between a metal and a salt. If we mix together sodium with magnesium sulfate, we will produce sodium sulfate (since sodium is more reactive and can displace magnesium) and pure magnesium. If you look at the symbol equation for this reaction, you can see that the sodium forms a positive ion when it forms the sulfate salt (remember that salts are ionic compounds made of positive and negative ions). Sodium loses electrons to form the positive ion, so we know that sodium has been oxidised, whereas magnesium gains electrons and is reduced.
Reactions of metals with acids
We can use the reactions of metals with acids to tell us how reactive that metal is. The more vigorously it reacts, the more reactive the metal. The slower the reaction (sometimes there is no reaction at all), the less reactive the metal.
Metals react with acids to form a salt and hydrogen, as described in the equation below:
You can use the following experimental set-up to determine the order of reactivity of different metals:
Add equal volumes of dilute hydrochloric acid or dilute sulfuric acid into a series of test tubes then add a equal mass of metal to each test tube. It is important that each metal has the same surface area because this will affect the rate of reaction.
Count the number of bubbles produced in a given time. The bubbles are hydrogen gas and can be confirmed using a lit splint, which will produce a ‘squeaky pop’ when the hydrogen burns.
The faster the bubbles are given off, the faster the rate of reaction and the more reactive the metal.
Let’s say we carry out the experiment described above by adding potassium, magnesium and zinc into test tubes containing dilute sulfuric acid. We’d expect the potassium to react explosively (in reality you would never do this in a school laboratory as it is too dangerous), while the magnesium would bubble vigorously and the zinc would form bubbles very slowly. We can therefore confirm their order of reactivity (from most reactive to least) as: potassium, magnesium then zinc.
Reaction of metals with water
We can also use the reaction of metals with water to determine order of reactivity. The equation for this reaction is:
Reactive metals (such as potassium, sodium, lithium and calcium) will react rapidly in cold water
Less reactive metals (such as magnesium, zinc and iron) won’t react with cold water but will react with water vapour (steam)
Unreactive metals (such as copper, silver and gold) won’t react with either cold water or steam
Extraction of metals by reduction
Unreactive metals, such as silver and gold, can be found in the Earth’s crust in a pure form, uncombined to other elements. However, more reactive metals, such as aluminium and magnesium, will usually be found combined to another element in a compound. An ore is a rock that contains enough of the metal to make it economically worth extracting.
The method of extracting a metal depends on its position in the reactivity series.
If a metal is less reactive than carbon, it can be extracted by reacting it with carbon in a displacement reaction. In a displacement reaction, the more reactive metal is able to take the place of a less reactive metal in a compound, since the more reactive metal forms stronger bonds. Carbon replaces the less reactive metal in a redox reaction, where the carbon is oxidised and the metal is reduced. We therefore refer to this method as reduction using carbon.
For example, copper is below carbon in the reactivity series so it is extracted from copper oxide by reacting it with carbon, to form pure copper and carbon dioxide. The copper loses oxygen so it is reduced while the carbon gains oxygen so it is oxidised. That’s why we call this method reduction with carbon – because the element that we are purifying is reduced.
Elements which are more reactive than carbon will be extracted using electrolysis. Aluminium is more reactive than carbon so it must be extracted from ores containing aluminium oxide using this method. Electrolysis uses electricity to separate the metal from the other elements in the compound.
Oxidation and reduction (triple science)
We’ve seen that oxidation is the gain of oxygen and reduction is the loss of oxygen during a chemical reaction. We can also define oxidation as the loss of electrons and reduction as the gain of electrons that takes place during a reaction. You can use the anagram OILRIG to help you remember.
For example, let’s consider the reaction between sodium and chlorine to form sodium chloride.
Sodium loses an electron to form a positive sodium ion. It has been oxidised.
Chlorine gains an electron to form a negative chloride ion. It has been reduced.
Did you know…
The surface of mars is red because it contains a high amount of iron on its surface. The iron reacts with oxygen to form rust, giving mars it’s reddish colour. Our blood has a dark red colour for the same reason - haemoglobin contains iron ions which bind to oxygen, enabling red blood cells to transport the oxygen around our body. This forms iron oxide, giving blood it’s deep red colour.
Next Page: Reaction of Acids