Structure and Bonding of Carbon
Carbon can bond with itself to form huge molecular structures. Depending on the way that it bonds with itself, carbon can form very different substances with some amazing properties. If it forms 4 bonds, you get a sparkly diamond, whereas if it forms 3 bonds you get the graphite we use in pencils. If it rolls itself into a ball, you get the nanoparticles that are useful in electronics.
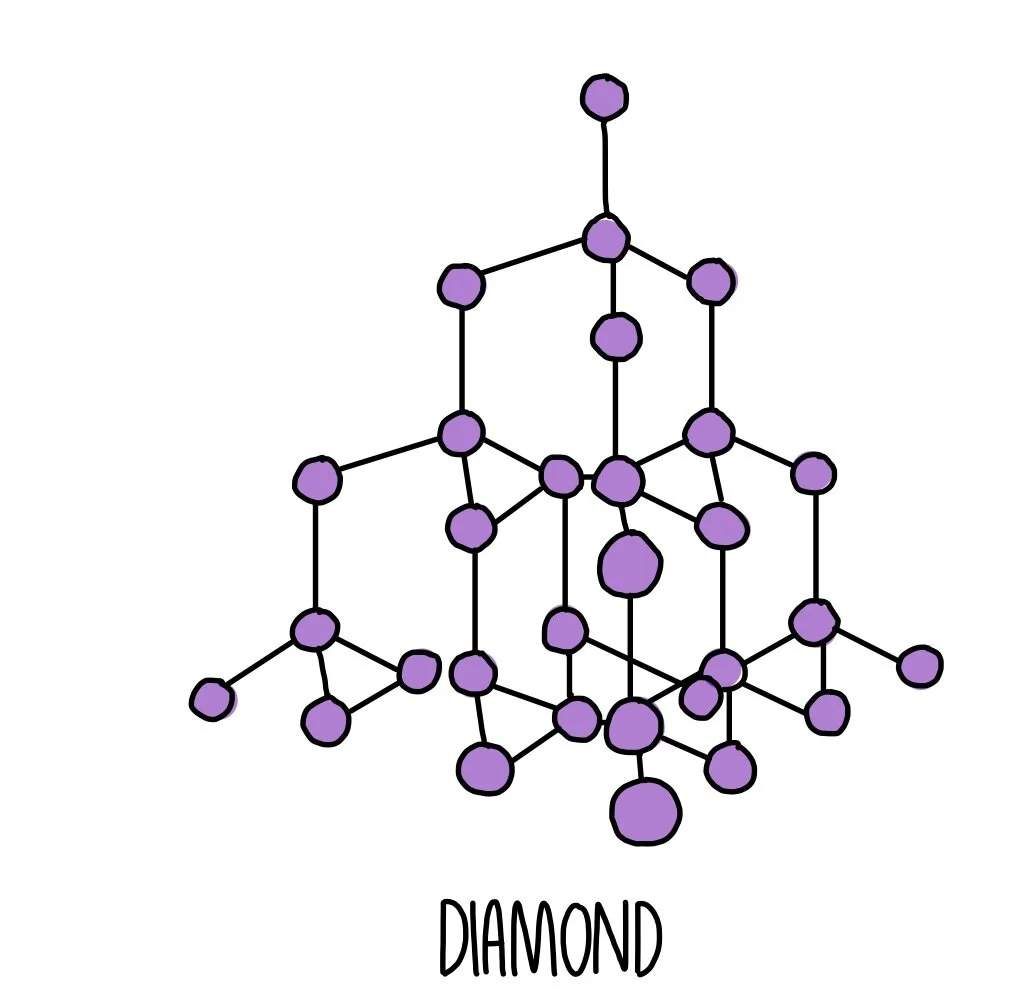
Diamond
Diamond is a giant covalent structure made up of carbon atoms bonded to four other carbon atoms. These atoms are held together by covalent bonds, making diamond very hard and giving it a high melting and boiling point. It is not able to conduct electricity because it does not have any charged particles which are free to move.
Graphite
Graphite is another giant covalent structure, but unlike diamond, each carbon atom is bonded to only three others in a planar hexagonal arrangement. This means that one of carbon’s outer electrons is not being used and it instead becomes delocalised, which means that it can move through the structure. Because these electrons are free to move and carry a charge, this means that graphite is able to conduct electricity. Since the carbon atoms are held together by strong covalent bonds, this means that graphite has a high melting and boiling point.
Graphite exists in layers of hexagons which are held together by weak intermolecular forces. These forces break easily, separating the layers from each other as they slide over each other. It is this property that makes graphite a useful substance in pencils – when you apply pressure and move the pencil, layers of graphite slide away, leaving a mark on the paper.
Graphene and Fullerenes
Graphene is just a single layer of graphite and so has similar properties – it is able to conduct electricity and has a high melting and boiling point. These properties mean that graphene is a useful material in electronics and composite materials.
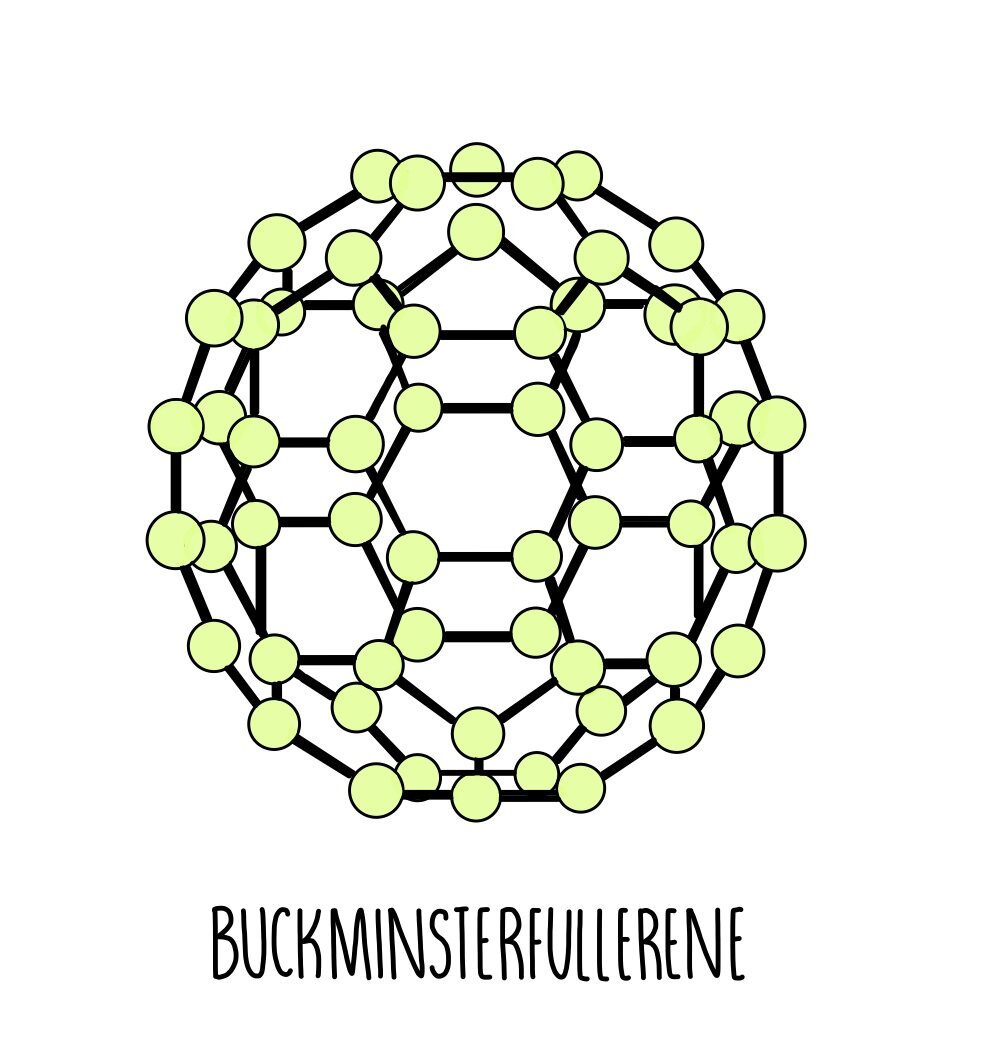
Fullerenes are molecules of carbon atoms with hollow shapes. The structure of fullerenes is based on hexagonal rings of carbon atoms but they may also contain rings with five or seven carbon atoms. The first fullerene to be discovered was Buckminsterfullerene which has a spherical shape and kind of resembles a beach ball. It is made up of 60 carbon atoms joined together by covalent bonds. There are weak intermolecular forces between Buckminsterfullerene molecules which are easily broken, meaning that it is slippery and has a low melting and boiling point.
Nanotubes are cylindrical fullerenes – they’re basically graphene rolled into a cylinder shape. They have high length to diameter ratios and are resistant to being stretched. Like graphene and graphite, they contain delocalised electrons so can conduct electricity. These properties make them a useful material for things like nanotechnology and electronics.
Did you know…
Scientists are currently researching the outer layer of portabella mushrooms as a material to replace graphite in lithium batteries. Their porous structure makes an ideal material for making efficient batteries to power smartphones and electric vehicles. Using mushrooms provides an environmentally-friendly, cheap and simple alternative to the synthetic graphite which is currently used.
Next Page: Nanoparticles