The Halogens
Properties of the halogens
- The halogens are the elements in group 7 of the periodic table.
- They all have seven electrons in their outer shells and form negative ions with a -1 charge.
- Since they gain an electron to achieve a full outer shell, halogens are reduced in redox reactions.
- They all have an outer shell electron configuration of s2 p5.
- In their neutral state, they exist as diatomic molecules (two atoms covalently bonded together e.g. Br2).
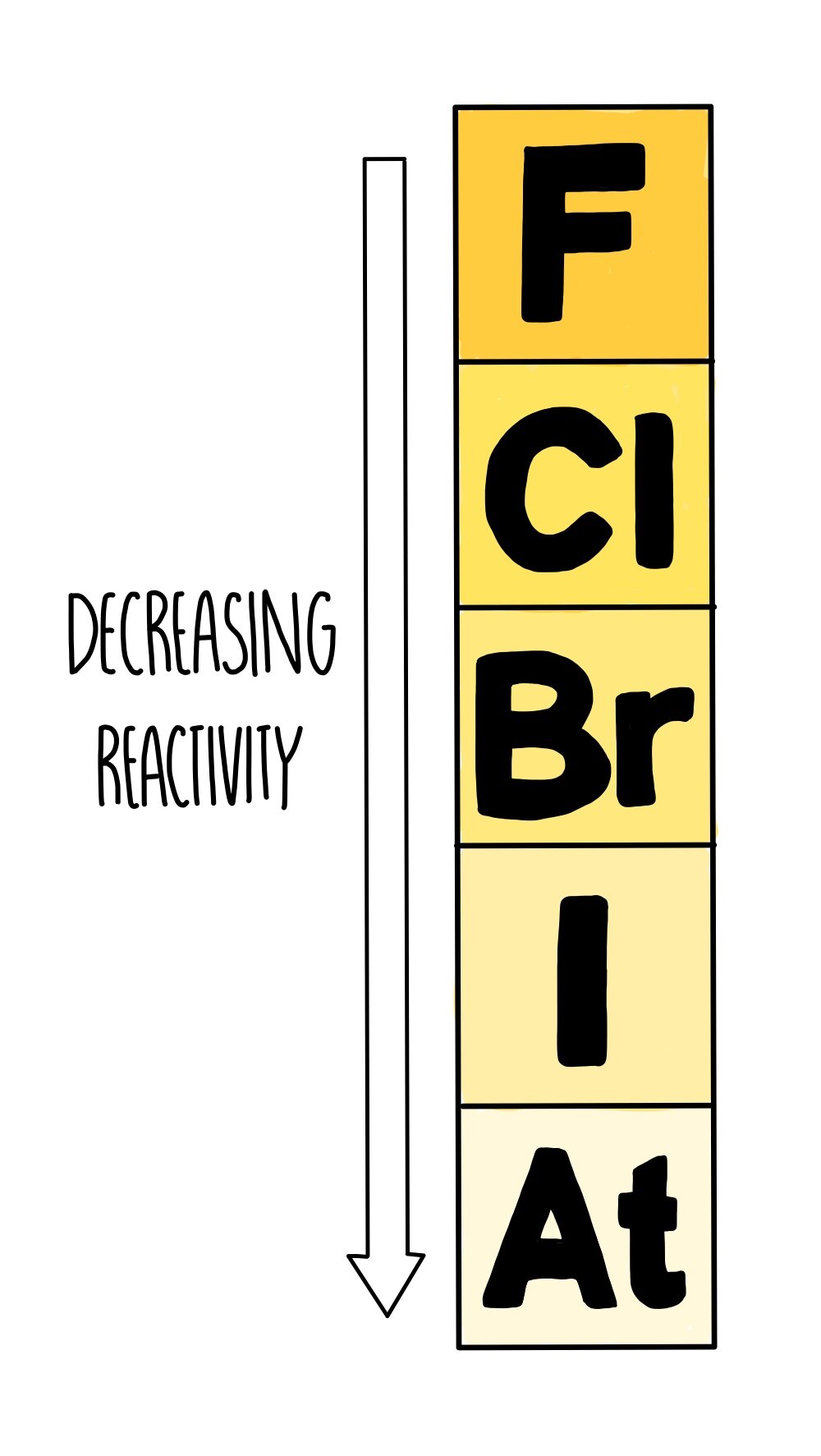
As you go down the group, their melting and boiling point increases — so the elements at the top of the group (fluorine and chlorine) exist as gases at room temperature, whereas bromine is a liquid and the elements at the bottom (iodine, astatine) are solids.
Melting and boiling points increase down the group because:
The number of electrons increases, so they form stronger London forces with other molecules.
The stronger London forces need more energy to overcome.
Reactivity
As you go down the group, the reactivity of halogens decreases.
This is because:
The outer electrons are further away from the nucleus, so the attraction between the nucleus and outer electron shell decreases.
There is more electron shielding, which further decreases the nuclear charge.
This makes it harder to grab hold of an additional electron and place it in the outermost shell, resulting in a reduction in reactivity from fluorine to iodine.
You can work out the order of halogen reactivity by carrying out a displacement reaction, since a more reactive halogen will be able to replace a less reactive halogen in a compound but not vice versa.
For any element in group 7, it will be able to displace any halogen that is beneath it in the periodic table.
For example, if you mix bromine water with potassium iodide, we’d expect the bromine to displace iodine because it is more reactive. We end up with iodine and potassium bromide. Bromine water is yellowy-orange, so we will observe a colour change from yellow/orange to colourless.
But no reaction takes place if you mix bromine water with potassium chloride. Bromine is beneath chlorine in group 7 so it is less reactive and unable to replace it in the compound. The reaction mixture will remain a yellow/orange colour.
To make the colour changes of halogen displacement reactions easier to see, we can add an organic solvent (e.g. hexane) to the reaction mixture. The halogen that is present will dissolve in the organic solvent, settling out as a distinct layer above the aqueous solution. Here’s what colour each halogen will appear as in organic solvent:
Chlorine – pale yellow-green
Bromine – orange
Iodine – purple
Test for halide ions
To test for halide ions, add dilute nitric acid and silver nitrate. Nitric acid removes any impurities that might obscure the result. Silver nitrate then reacts with any halide ions to form coloured silver halide precipitates.
Think milk, cream, butter
Silver chloride is a white precipitate
Silver bromide is a cream precipitate
Silver iodide is a pale yellow precipitate
The issue with this test is that the colours of the precipitates are subjective. To confirm our result, we can carry out an additional test involving ammonia solution.
The three precipitates have different solubilities in aqueous ammonia:
Silver chloride dissolves in dilute ammonia solution
Silver bromide will only dissolve in concentrated ammonia solution
Silver iodide will not dissolve in dilute or concentrated ammonia solution (it is insoluble)
Disproportionation reactions
Disproportionation is when the same element is simultaneously oxidised and reduced.
For example, in the manufacture of bleach, chlorine gas is mixed with cold, dilute aqueous sodium hydroxide. Sodium hypochlorite (NaClO), or bleach, is formed, along with sodium chloride and water.
Assigning oxidation numbers to the reactants and products, we can see that chlorine is being both oxidised and reduced.
- Cl goes from 0 in Cl2 to +1 in NaClO therefore it is being oxidised
- Cl goes from 0 in Cl2 to -1 in NaCl therefore it is being reduced
Since oxidation and reduction are happening to the same element in the same reaction, disproportionation is taking place.
Uses of chlorine
Chlorine is used to make bleach, a useful household disinfectant.
It’s also added to water to make it safe to drink — it kills any microorganisms in the water and prevents millions of people suffering from diseases such as cholera and typhoid.
The equation below shows what happens when chlorine is added to water. This is another example of a disproportionation reaction.
Toxicity
However, just like it kills bacteria, it can also kill humans if it is concentrated enough.
Chlorine gas is toxic and can irritate the respiratory system if inhaled.
Liquid chlorine can burn the skin and irritate the eyes, causing serious injuries.
When chlorine reacts with hydrocarbons, it forms chlorinated hydrocarbons which can cause cancer if they make their way into the human body (they are carcinogenic).