The Periodic Table
The periodic table is how chemists have organised the known elements on Earth. The elements are arranged in columns called groups - all of the elements in the same group will take part in the same kind of chemical reactions.
The periodic table
Elements in the periodic table are arranged in rows (periods) in order of increasing atomic number, with each successive element containing one extra proton compared to element before them. The table is arranged in columns called groups containing elements which have similar chemical properties. The groups are numbered from one to eight, skipping out the block of elements in the middle of the table.
Elements in the same group in the periodic table have the same number of electrons in their outer shell (outer electrons) and this gives them similar chemical properties. For example, fluorine is in group 7 of the periodic table so we know that it has seven electrons in its outer shell. Other elements in group 7 include chlorine, bromine and iodine – all of these elements share similar properties and will react in similar ways.
Most of the Periodic Table consists of metallic elements, located on the left hand side of the table, with the non-metallic elements on the right.
We can use the position of elements in the periodic table to predict the reactions they will take part in:
Elements in groups 1 and 2 (which have either 1 or 2 electrons that they need to lose to get a full outer shell) will react with elements in groups 6 or 7 when they form compounds.
Elements in group 8 (the noble gases) will not react with other elements because they already possess a complete octet
Development of the Periodic Table
Before scientists knew about subatomic particles (protons, neutrons and electrons), elements were organised in order of their atomic weights (i.e. increasing mass numbers). These early periodic tables were incomplete and meant that some elements were placed in groups with other elements which had very different chemical properties.
A Russian chemist called Dmitri Mendeleev overcame this by leaving gaps in the table and swapping elements around so that they were placed in groups with other similar elements. In later years, elements were discovered which fit in the gaps that Mendeleev placed in his periodic table. Once scientists fully understood the concept of isotopes, they realised why certain elements needed to be swapped over to fit in their correct positions in the table.
The modern periodic table is organised in increasing atomic number (i.e. the number of protons in the nucleus) instead of by increasing mass number.
Metals and Non-Metals
Most elements are metals and are found on the left hand side of the periodic table. When metals react they lose electrons to form positive ions. Non-metals are much fewer in number and are located on the right hand side of the periodic table. Non-metals tend to gain electrons when they react to form negative ions, or share electrons with other non-metals to form covalent compounds.
Metals have the following properties:
Form positive ions
Conduct heat and electricity
Shiny in appearance
Their oxides act as bases
High density
Malleable (can be bent or hammered into shape)
Non-metals share the following properties:
Do not form positive ions
Do not conduct electricity
Dull in appearance
Their oxides are acidic
Low density
Brittle (snap easily when bent)
Group 0
Helium, like the other noble gases, are gaseous at room temperature
The last group of the periodic table is group 0 (sometimes also called group 8) and is made up of the noble gases. These elements are all gaseous at room temperature and have a complete outer shell of eight electrons, except helium which only has two electrons. Since these elements have a complete outer shell they are unreactive so do not form molecules or compounds.
As you go down group 0, from helium through to radon, the boiling point increases. This is because the elements have more and more electrons as you go down the group, which means that they form stronger intermolecular forces. These stronger intermolecular forces need more energy to overcome when the element is being converted from a liquid to a gas.
Group 1
Lithium, sodium and potassium all react vigorously with water to produce a metal hydroxide and hydrogen. The metal hydroxide is an alkali which simply a substance which release hydroxide ions (OH-) into solution. Because all of the group 1 metals react with water to form alkalis, they are given the name 'alkali metals'. The equation for the reaction of lithium with water is written below:
The group 1 metals react with oxygen in the air to form a metal oxide. The equation for the reaction of sodium with oxygen is written below:
You also need to know about the reaction of group 1 metals with chlorine. The group 1 elements will give their one outer electron to chlorine so that both elements have a complete outer shell. A chloride salt will be formed. For example, sodium reacts with chlorine to form the ionic compound sodium chloride.
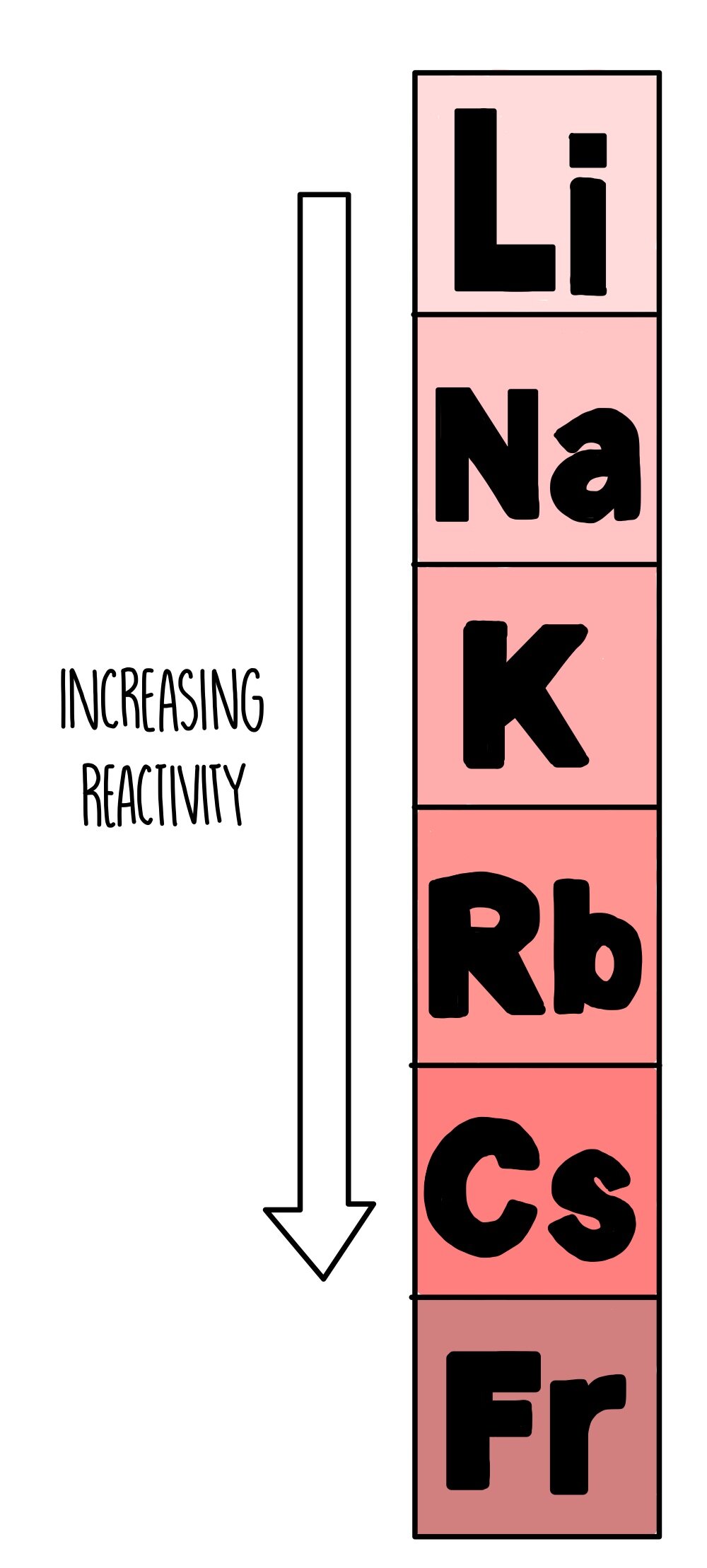
As you go down group 1, the reactivity of the metals increases. Lithium and sodium themselves are pretty volatile, but once you reach potassium with its fiery temper and serious anger management issues, you’re in for an explosion. The elements below potassium are even more reactive, but they aren’t nearly as common and only found in small amounts.
If you react each of the first three group 1 metals with water you should make the following observations:
Lithium fizzes slowly until it disappears
Sodium fizzes rapidly and disappears faster
Potassium burns violently with a purple flame and disappears extremely quickly
When the group 1 metals react, they form positive ions with a +1 charge by losing their single outer electron. As you go down the group, the number of electron shells increases so the electron that needs to be lost is further away from the nucleus. The greater the distance between the electron and the nucleus, the weaker the attraction between the nucleus and the electron. Since the nucleus doesn’t have such a tight grip on this outer electron, it is more easily taken by another atom to form a positive ion.
Group 7
The halogens are the elements found in the second-to-last group of the Periodic Table. They all have seven electrons in their outer shell and since they need only one more to complete their octet they are fairly reactive. The halogens typically form ionic compounds with elements in group 1 which have one outer electron that the group 7 elements can’t resist taking.
As you go down the halogens, from fluorine to astatine, the elements become darker in colour and have a higher boiling point. Boiling point increases as you go down the group because the mass of each element increases and they have more electrons around their nuclei. The more electrons an element has, the more intermolecular forces (London dispersion forces) it can form. Each halogen has the following characteristics at room temperature:
Fluorine is a pale yellow gas
Chlorine is a poisonous green gas
Bromine is a toxic red-brown liquid
Iodine is a dark grey solid which gives off a purple vapour when heated
Astatine is a black solid
Unlike the group 1 metals, reactivity decreases as you go down the halogens. This means that fluorine, at the top of the group, is the most reactive. Fluorine is so eager to react with anything that it is almost never found as a pure element and it is so dangerous to work with that scientists avoid handling it in reactivity experiments. Chlorine is less reactive and much more manageable, and added to water in small quantities to kill microorganisms to make it safe to drink.
The group 7 elements want to gain one more electron so that they have a stable electronic structure. The smaller the atom, the easier it is to grab an electron from another atom, making the atom more reactive. As you go down group 7, the atomic radius increases and it becomes more difficult to attract another electron.
In displacement reactions, a more reactive element will displace (replace) a less reactive one. Think of it as the introduction of an attractive geordie on love island, forcing someone else out of a relationship and leaving them by themselves. Displacement reactions involving halogens and halogen ions (halides) can be used to provide evidence for the order of reactivity of the halogens.
An example is the addition of chlorine to a solution of potassium iodide. Chlorine is more reactive than iodine so will take its place in the molecule, resulting in the formation of potassium chloride and iodine. The equation for the reaction would look like this:
Let’s say we add iodine to a solution of potassium bromide. This time we’re adding an element which is less reactive than the halogen in the compound so no reaction will take place.
Did you know…
Iodine is linked to intelligence. It’s found naturally in fish and dairy foods and is used in the body to make thyroid hormone which is involved in brain development. In some parts of the world where iodine levels in the soil are low, or fish is not part of the diet, iodine deficiency has been linked with low intelligence. In most parts of the world iodine is now added to salt.
Next Page: Transition Metals