The periodic table
In 1869 a Russian scientist called Dimitri Mendeleev published his periodic table. With just a few adjustments, the modern Periodic Table became one of the most important tools in chemistry.
Organisation of the periodic table
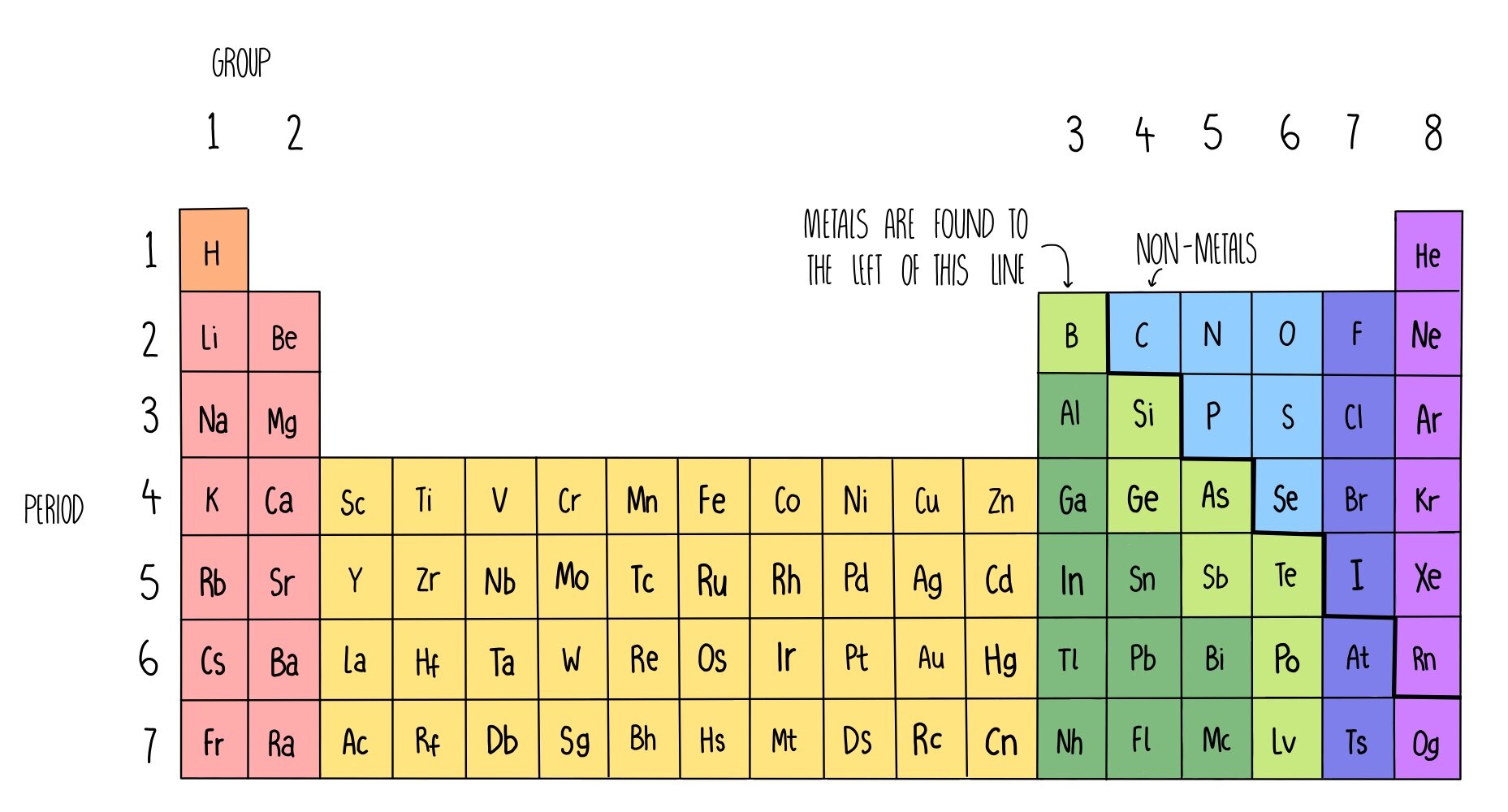
Elements in the Periodic Table are arranged in rows (periods) in order of increasing atomic number, with each successive element containing one extra proton compared to element before them. The table is arranged in columns called groups containing elements which have similar chemical properties. The groups are numbered from one to eight, skipping out the block of elements in the middle of the table.
Most of the Periodic Table consists of metallic elements, located on the left hand side of the table, with the non-metallic elements on the right.
We can use the position of elements in the periodic table to predict the reactions they will take part in:
Elements in groups 1 and 2 (which have either 1 or 2 electrons that they need to lose to get a full outer shell) will react with elements in groups 6 or 7 when they form compounds.
Elements in group 8 (the noble gases) will not react with other elements because they already possess a complete octet
Development of the periodic table
Before scientists knew about subatomic particles (protons, neutrons and electrons), elements were organised in order of their atomic weights (i.e. increasing mass numbers). These early periodic tables were incomplete and meant that some elements were placed in groups with other elements which had very different chemical properties.
A Russian chemist called Dmitri Mendeleev overcame this by leaving gaps in the table and swapping elements around so that they were placed in groups with other similar elements. In later years, elements were discovered which fit in the gaps that Mendeleev placed in his periodic table. Once scientists fully understood the concept of isotopes, they realised why certain elements needed to be swapped over to fit in their correct positions in the table.
The modern periodic table is organised in increasing atomic number (i.e. the number of protons in the nucleus) instead of by increasing mass number.
Electronic configuration
An atom will have the same number of electrons as the number of protons in its nucleus — so the amount of electrons will be the same as its atomic number. Since each successive element in the periodic table has one more proton than the last, they also have an additional electron.
Electrons occupy specific energy levels around the nucleus which can only hold a fixed number of electrons. The innermost energy level holds a maximum of two electrons then the following energy levels can hold eight electrons each. Just like collecting Pokemon cards as a kid, atoms are always eager to get a complete set and will share, steal or throw away electrons until their electron shells are completely occupied with electrons.
To represent electron configuration we write the number of electrons in each energy level, separated by commas. For example:
Magnesium has 12 electrons with an electron configuration of 2, 8, 2.
Chlorine has 17 electrons with an electron configuration of 2, 8, 7.
Notice that the last number is always the same as the group number that element is found in. Magnesium is in group 2 and has two outer electrons whereas chlorine is in group 7 so has seven outer electrons. The number of electrons in the outer shell determines how an element reacts. Therefore, all elements found in the same group have similar chemical properties because they have the same number of outer electrons.
The last group on the Periodic Table are the Noble gases. These have a complete outer energy level with eight electrons in their outer shell. A full energy level makes these elements very stable, with no need to take or give away electrons to other elements, therefore these elements are very unreactive.
Metals vs non-metals
Most elements are metals and are found on the left hand side of the periodic table. When metals react they lose electrons to form positive ions. Non-metals are much fewer in number and are located on the right hand side of the periodic table. Non-metals tend to gain electrons when they react to form negative ions, or share electrons with other non-metals to form covalent compounds.
Metals have the following properties:
Form positive ions
Conduct heat and electricity
Shiny in appearance
Their oxides act as bases
High density
Malleable (can be bent or hammered into shape)
Non-metals share the following properties:
Do not form positive ions
Do not conduct electricity
Dull in appearance
Their oxides are acidic
Low density
Brittle (snap easily when bent)