Using Materials
Metals corrode when they react with substances in the environment, so we may protect them by painting or galvanising the metal. We also combine metals with other elements to modify its properties.
This topic is only included on the AQA triple science specification.
Corrosion
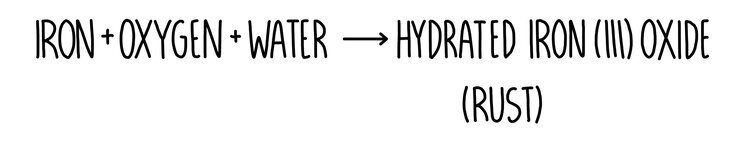
Corrosion is when metals react with substances present in the environment, causing the metal to be destroyed. Rusting is an example of corrosion and occurs when iron reacts with oxygen and water in the air. When iron reacts with oxygen and water, hydrated iron oxide is formed. This is rust and has an orange-reddish colour.
Rust is formed when iron reacts with water and oxygen.
Corrosion can be prevented by applying a coating to the surface of the metal, such as a layer of grease, paint or electroplating. This acts as a barrier and stops the metal underneath from coming into contact with reactive substances in the environment. For example, aluminium has an outer oxide layer which protects it from corroding further. Some coatings work by containing a metal that is more reactive than the one you want to protect. The more reactive metal will react with the substances in the environment instead of the metal underneath – this is called sacrificial protection. For example, galvanisation is a method whereby iron is covered in a zinc coating to prevent rusting. Since zinc is more reactive than iron, the zinc will react with any oxygen or water present in the environment, preventing iron from reacting to form rust.
Alloys
Alloys are mixtures of metals with other elements. Adding another element to a metal disrupts its structure, making it harder and stronger.
Alloys may have additional useful properties that are absent in the pure metal. For example:
Aluminium alloys have a lower density than pure aluminium so are a useful material for aeroplanes.
Bronze is an alloy of copper and tin
Brass is an alloy of copper and zinc
The gold used in jewellery is usually an alloy of silver, copper and zinc. The amount of actual gold in gold jewellery is measured in carats. Pure gold is classed as 24 carat, whereas 50% gold is 12 carat.
Steel is an alloy of iron and carbon. The properties of steel can be altered by varying the proportion of carbon it contains. High carbon steel is strong but brittle, which means it can break easily when bent. Low carbon steel is malleable (can be easily shaped without breaking) but is soft. Stainless steels contain additional metals (chromium and nickel) which make them resistant to corrosion and useful for things like dishwashers and cutlery.
Aluminium is alloyed with other elements to make it more lightweight. Aluminium alloys are used for things like bikes, cars and aeroplanes.
Ceramics, polymers and composites
Glass has some useful properties – it is hard and transparent so makes a very good material for windows. However, it is brittle which means it can break easily. Most of the glass we use is soda-lime glass which is made from mixing sand (silicon oxide), sodium carbonate and limestone at high temperatures then allowing the molten mixture to cool and solidify. Borosilicate glass is made by mixing sand and boron trioxide. Borosilicate glass melts at higher temperatures than soda-lime glass.
Clay ceramics, such as bricks and pottery, are made by shaping wet clay and then heating in a furnace. This causes crystals to form and join together. They are then covered in a glaze which makes the surface hard and waterproof.
Polymers are long-chain molecules formed when lots of smaller molecules (monomers) join together. The properties of polymers depends on what monomers they are made of the and the conditions under which they are made. For example, depending on the conditions, ethene can form two types of polymer: low density polyethene (LDPE) and high density polyethene (HDPE). LDPE is unreactive and flexible, making it a good material for plastic bags and bubble wrap. HDPE is harder and resistant to chemical attack, making it suited for buckets, plastic piping and plastic bottles. The different properties of LDPE and HDPE is due to their structures. The polymer chains of the low density form are branched which means that the molecules push each other apart and are arranged randomly. The high density form has only straight chain polymers which can pack closely together.
Thermosoftening polymers melt when they are heated. This is useful because it means that they can be melted and remoulded into other products when they are recycled. These plastics are able to melt because there are no covalent bonds between the polymers, so the molecules can move over each other when heat is applied, causing the plastic to melt. Thermosetting polymers do not melt when they’re heated, making them much more resistant to high temperatures than thermosoftening plastics. Thermosetting plastics will be used for things like electrical plugs, which may be exposed to high temperatures if there is a problem with the internal wiring. The reason thermosetting polymers don’t melt is because there are strong covalent bonds between the polymers. This prevents the chains from sliding over each other when they are heated.
Composite materials are made by mixing two materials with different properties together. By mixing them together, it is possible to create a material with improved properties. Composite materials are made up of two basic components: the reinforcement and the matrix, which binds the reinforcement together. Reinforced concrete is an example of a composite material. It has a steel reinforcement and a concrete matrix. Another example of a composite material is fibreglass, which is made of a glass fibre reinforcement and polymer resin matrix. By combining these materials, you can create fibreglass which is strong, stiff and lightweight. Chipboard is another composite material made of wood chip reinforcement bound with a resin glue matrix. Mixing these materials together makes chipboard very strong.
Did you know…
Vinicunca mountain in Peru, also known as rainbow mountain for its colourful stripes, gets its colourful appearance from the metals present in the soil. Over time, sediments containing different proportions of mineral ions get pushed to the surface and react with elements in the environment to produce different colours. The reds come from iron oxide deposits while the yellows are due to sulfur compounds.
Next Page: Fertilisers