Water, ATP and Inorganic Ions
Water
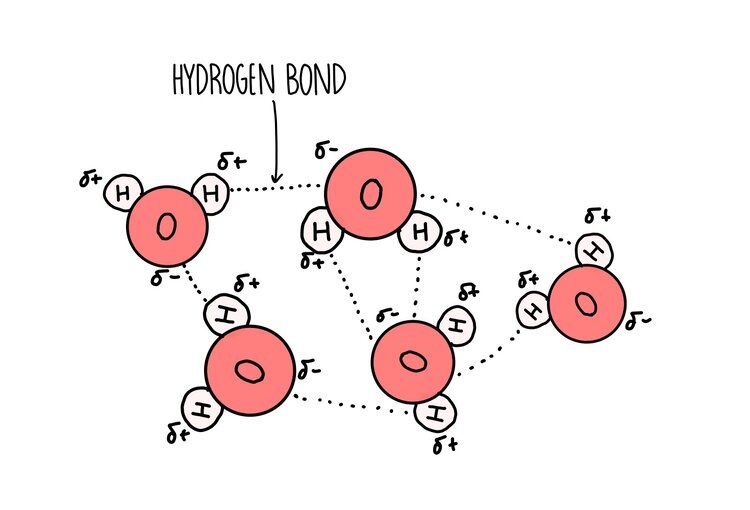
Water is a polar molecule - this means that the electrons are shared unequally within the bonds that hold a water molecule together. Oxygen is greedy when it comes to electrons and is able to pull the electrons closer towards it (we say it is more electronegative). This gives oxygen a slight negative charge and hydrogen a slight positive charge. A hydrogen bond forms between the slightly negative oxygen atom of one water molecule and the slightly positive hydrogen atom on another molecule. The ability of water molecules to hydrogen bond gives it some special properties:
It is an excellent solvent: water is a good solvent because it can form hydrogen bonds with other polar molecules or charged ionic compounds. Water dissolves more substances than any other liquid so is referred to as the ‘universal solvent’. This is handy for humans, since our blood consists mostly of water which is able to dissolve hydrophilic molecules such as glucose, ions and some amino acids.
It has a high latent heat of evaporation - this means that a lot of energy is used to convert water from a liquid to a gas. This is why we feel cooler when we sweat, since the evaporation of water from our skin’s surface takes a lot of heat energy with it.
It has a high specific heat capacity - all those hydrogen bonds within water are great at absorbing energy, which means you have to add a lot of it to heat water up. This is really useful for marine life since it means that bodies of water are fairly resistant to changes in temperature, making it a stable habitat in which to live.
Adenine triphosphate (ATP)
ATP is a phosphorylated nucleotide. Its structure consists of a ribose sugar and adenine attached to three phosphate groups. Hydrolysis of ATP to ADP removes one of the phosphate groups and releases energy in a single reaction. It also releases energy in small, manageable quantities which means less is wasted.
When ATP is hydrolysed, it is converted into adenine diphosphate (ADP) and inorganic phosphate (Pi). This reaction is catalysed by the enzyme ATP hydrolase.
As well as releasing energy, the phosphate can be attached to other molecules to make them more reactive. Enzymes and other proteins can be phosphorylated to convert them from an inactive to an active form.
ATP is re-synthesised in a condensation reaction, joining ATP and Pi to reform ATP. This reaction is catalysed by the enzyme ATP synthase.
Inorganic ions
There is an array of cations (positive ions) and anions (negative ions) which are necessary for many biological processes:
- Calcium ions, Ca2+: required for the transmission of action potentials, the formation of bone, the release of insulin from the pancreas and as an enzyme cofactor.
- Sodium ions, Na+: required for the generation of action potentials, muscle contraction and for maintaining blood pressure.
- Potassium ions, K+: required for the generation of action potentials, muscle contraction, maintaining blood pressure and for activating photosynthesising enzymes in plants.
- Hydrogen ions, H+: important role in respiration and photosynthesis reactions and influences pH of a cell by increasing acidity.
- Ammonium ions, NH4+: an important part of the nitrogen cycle. It is absorbed by plants and incorporated into amino acids for protein synthesis.
- Nitrate ions, NO3-: an important part of the nitrogen cycle. It is absorbed by plants and incorporated into amino acids for protein synthesis.
- Hydrogencarbonate ions, HCO3-: maintains constant pH in the blood by acting as a buffer.
- Chloride ions, Cl-: maintains constant pH in the blood through the process of 'chloride shift' and acts as a cofactor for the enzyme amylase.
- Phosphate, PO43-: used to synthesise biological molecules such as nucleic acids and phospholipids and is added to enzymes and other proteins to regulate their activity.
- Hydroxide, OH-: changes the pH of a cell by making the area more alkaline.