Atomic Structure and Electron Configuration
Atoms are ‘building blocks’ of matter. They can be split apart into three subatomic particles: protons, neutrons and electrons. We know now that protons and neutrons are located in the nucleus of the atom, with electrons spinning in orbitals outside the nucleus. It took a while for scientists to understand this and models of atomic structure have gradually developed as more evidence has become available.
Atoms, Elements and Compounds
All substances are made of atoms. An atom is the smallest part of an element that can exist. Atoms of each element are represented by a chemical symbol, e.g. N represents an atom of nitrogen and K represents an atom of potassium. There are about 100 different elements, each of which has a place on the Periodic Table.
Atoms of elements react in chemical reactions to form compounds – remember that a compound is made up of atoms from two or more elements bonded together, such as sodium chloride, NaCl. The atoms which make up a compound can only be separated by chemical reactions. During a chemical reaction, at least one new product is formed. Chemical reactions are also associated with an energy change (e.g. a release of heat energy or absorption of heat energy).
You may be expected to come up with the formula of an ionic compound. To do this, all you need to do is figure out the charges of the ions which make it up then use the crossing over method to work out the formula of the whole compound.
For example, to work out the formula of the compound magnesium chloride, we first write down the charges of the magnesium ion and the chloride ion. Magnesium has a +2 charge, since it is in group 2 of the Periodic Table so needs to lose two electrons to form a stable ion. Chloride ions have a -1 charge, since they are in group 7 so need just one more electron to achieve a stable octet. Then we ‘cross-over’ the charges to get the formula of the whole compound, MgCl2.
You’ll also be expected to be able to balance chemical equations. The easiest way to do this is to count the number of each atom on the left and right hand side of the equation. Remember that you need to multiply the number of atoms by any little numbers written after it. If you have a group of atoms written in brackets, you need to multiply all of the atoms in the brackets by the number that comes after them.
Look at the example below to see how you would balance the reaction between calcium hydroxide and sulfuric acid to form calcium sulfate and water.
If you are doing higher tier, you may also be asked to write an ionic equation. This is different from a full equation as we can exclude any ions that don’t change in the conversion of reactants to products. For example, in the reaction of aluminium with hydrochloric acid to form aluminium chloride and water, we would carry out the following steps:
Add state symbols to your reactants and products – either solid (s), liquid (l), aqueous (aq) or gas (g). Remember that water is always a liquid and a soluble salt will be aqueous.
Anything with the symbol aq can be written out as the ions which make it up. E.g. hydrochloric acid can be written as hydrogen ions (H+) and chloride ions (Cl-).
Now cancel out any ions which are the same on the left and right hand side of your equation. In our example below, aluminium ions (Al3+) are present in both the reactants and products, so we can remove these, leaving us with our final ionic equation.
Mixtures
A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. These processes do not involve chemical reactions so the elements and compounds are not changing, they are just being separated from each other.
Filtration
Filtration can be used to separate an insoluble solid from a liquid, such as removing sand from a mixture of sand and water. It is carried out by lining a funnel with a sheet of filter paper and pouring the mixture into the funnel. The filter paper contains tiny holes, which are small enough to allow the liquid to pass through but will retain any solid particles. The liquid which is collected is called the filtrate.
Simple distillation
Simple distillation is used to separate a liquid from a solution. For example, it could be used to remove water from a mixture of salt and water. The solution is placed in a flask and heated from underneath. When the solvent evaporates, it passes into a condenser which causes the condensation of the solvent gas into a liquid, which can then be collected in a beaker.
Fractional distillation
Fractional distillation is a similar technique to simple distillation, but is used to separate multiple liquids from a mixture. It is used in the separation of different liquid fuels from crude oil, which works because each liquid has a different boiling point. The crude oil is vaporised and passed into a fractionating column, which is hot at the bottom and cool at the top. The molecules float up the column and condense when the temperature inside the column equals their boiling point. The liquids are removed at different heights along the column and each have slightly different uses.
Crystallisation
Crystallisation is used to form solid crystals from a solution, such as crystals of copper sulfate from copper sulfate solution. To do this, the solvent is slowly evaporated by gently heating the solution. The remaining solution is left to cool to allow the crystals to form and any remaining solution is filtered off. The crystals are washed and dried (either in a warm oven or air-dried).
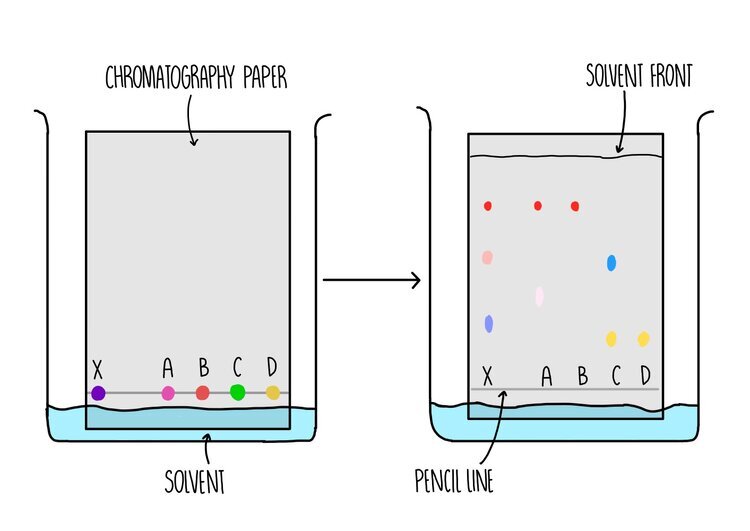
Chromatography
Paper chromatography is used to separate a mixture of soluble substances, such as separating dyes within food colouring or plant pigments. It involves a stationary phase (the part of the equipment which doesn’t move) which is a sheet of paper and a mobile phase (the thing which moves) which is the solvent.
Method:
A line is drawn near to the bottom of the piece of paper - this is the starting line where the dyes will be placed. It is important that the line is drawn in pencil because the ink from pens will also dissolve in the solvent and cause a mess.
The paper is placed in a tank containing some solvent and a lid is placed on top of the tank to prevent evaporation of the solvent. The solvent need to be below the starting line so that the dyes do not dissolve in the solvent before they can move along the paper.
As the paper absorbs the solvent, the solvent moves further up the stationary phase. The dyes are carried up the paper along with the solvent with different dyes moving up the paper to different extents. The total distance travelled by the solvent is the solvent front.
An Rf value is calculated for each dye by dividing the distance travelled by the dye by the distance travelled by the solvent (the solvent front).
Models of the atom
The plum pudding model of the atom
Science is not static – we’re constantly updating scientific models as new research and evidence becomes available. This is the case for the model of atomic structure. Before the electron was discovered, atoms were thought to be the smallest things that existed. When the electron was identified, scientists realised that the atoms could be divided into even smaller subatomic particles, leading to the plum pudding model of the atom. This model suggested that the atom exists as a ball of positive charge with negative electrons scattered in it, like raisins in a plum pudding.
In 1905, Ernest Rutherford carried out an experiment which disproved the plum pudding model. His experiment involved shooting a beam of alpha particles at a thin piece of gold foil suspended in a vacuum. If the plum pudding model was correct, the alpha particles should all be repelled from the atoms in the gold foil, since the atom was thought to be positively charged and alpha particles are also positively charged (remember that like charges repel each other). Instead, Rutherford found that most of the alpha particles passed straight through the gold foil, with only a very small number being repelled. This led him to conclude two things:
The atom is mostly empty space, with a tiny nucleus located in its centre
The nucleus of the atom is positively charged
As a result of Rutherford’s findings, the nuclear model of the atom was proposed which replaced the plum pudding model. The nuclear model suggests that the atom is made up of a small central nucleus with electrons whizzing around it.
The nuclear model was updated when Niels Bohr discovered that electrons don’t move around just anywhere outside the nucleus but occupy specific energy levels (orbitals). The new atomic model was similar to the nuclear model, except that the electrons moved in orbitals at fixed distances around the nucleus.
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles. The experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus. This was about 20 years after the nucleus became an accepted scientific idea.
Size and charge of subatomic particles
The nucleus is made up of protons and neutrons, which are the heaviest particles. Electrons are approximately 2000 times lighter than a proton or neutron and weigh practically nothing, therefore most of the mass comes from the tiny nucleus. Protons are positively charged whereas neutrons have no charge, making the nucleus positively charged overall. Electrons are negatively charged, which enables the nucleus to keep hold of its electrons, due to the attraction between the opposite charges. In an atom, the number of electrons is equal to the number of protons in the nucleus. Atoms have no overall electrical charge.
Each atom has an atomic number and a mass number, which are displayed on the Periodic table as the small number and larger number next to each chemical symbol. The atomic number tells us the number of protons which is unique for each element. If the number of protons changes (and therefore the atomic number changes), which could occur during radioactive decay, for example, then the identity of the element also changes. The mass number tells us the number of both protons and neutrons in the nucleus. Remember that most of an atom’s mass comes from the nucleus and that the nucleus is made up of both protons and neutrons. Two atoms of the same element can have different mass numbers because they are isotopes.
Relative Atomic Mass
Isotopes are atoms which have the same number of protons but different numbers of neutrons. Therefore they have the same atomic number but a different mass number. For example, the most common isotopes of carbon are carbon-12 and carbon-13, in which carbon-13 has an additional neutron in its nucleus to give it a higher mass number.
The mass numbers that we see in the periodic table are an average of all the masses of all isotopes of a particular element. We call this the relative atomic mass (RAM) and can be calculated using the following formula:
Electronic Structure
An atom will have the same number of electrons as the number of protons in its nucleus, which means the amount of electrons will be the same as its atomic number. Since each successive element in the periodic table has one more proton than the last, they also have an additional electron. Electrons occupy specific energy levels around the nucleus which can only hold a fixed number of electrons. The innermost energy level holds a maximum of two electrons then the following energy levels can hold eight electrons each. Just like collecting Pokemon cards as a kid, atoms are always eager to get a complete set and will share, steal or throw away electrons until their electron shells are completely occupied with electrons.
To represent electron configuration we write the number of electrons in each energy level, separated by commas. For example:
Magnesium has 12 electrons with an electron configuration of 2, 8, 2.
Chlorine has 17 electrons with an electron configuration of 2, 8, 7.
Notice that the last number is always the same as the group number that element is found in. Magnesium is in group 2 and has two outer electrons whereas chlorine is in group 7 so has seven outer electrons. The number of electrons in the outer shell determines how an element reacts. Therefore, all elements found in the same group have similar chemical properties because they have the same number of outer electrons.
The last group on the Periodic Table are the Noble gases. These have a complete outer energy level with eight electrons in their outer shell. A full energy level makes these elements very stable, with no need to take or give away electrons to other elements, therefore these elements are very unreactive.
Did you know…
Francium, element number 87 on the Periodic Table, is so rare that at any one time there are only about 20 grams on the planet. It is so unstable that it has a half life of just 22 minutes.
Image credit: Slate
Next Page: The Periodic Table