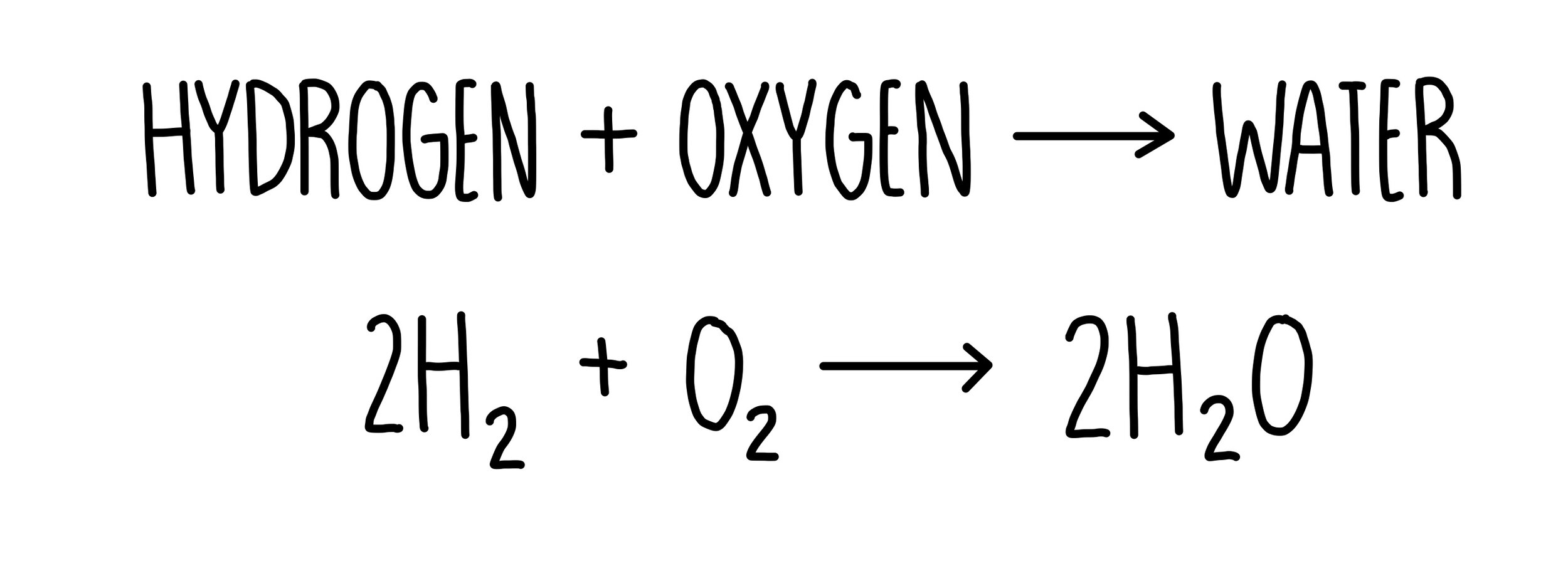Fuel Cells
If you’ve learnt about electrolysis already, you’ll know that we can use electricity to generate chemical reactions, separating an ionic compound. Fuel cells use the opposite principle: using chemical reactions to generate electricity.
This topic is only included on the AQA triple science specification.
Cells and batteries
A cell is the proper word for what you probably think of as a battery – the cylindrical objects that power TV remotes and other devices. Cells contain chemicals in the form of a solid metal (electrode) and an ionic solution (the electrolyte), which react and generate electricity by releasing electrons. The voltage produced by a cell depends on the type of electrode and electrolyte used. You can make a simple cell by connecting two different metals in contact with an electrolyte solution.
A battery is two cells joined together in series to increase the voltage. Batteries can be rechargeable or non-rechargeable. In non-rechargeable cells or batteries (such as alkaline batteries), the reactants will eventually be used up and the reaction will stop. With rechargeable cells and batteries, an external electric current reverses the reaction once the reactants have been used up. This ensures that the reaction can keep going and the battery can continue to supply electricity.
Fuel cells
Fuel cells differ from chemical cells in that they generate a continuous voltage, providing they have a fuel supply (such as hydrogen) and oxygen. Within the fuel cell, the fuel is oxidised electrochemically rather than being burned. This means that the reaction takes place at a lower temperature than a typical combustion reaction. Energy is released as electrical energy.
The overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water.
We can break down this overall reaction into two half equations, one of which involves oxidation at the anode and the other involves reduction at the cathode.
Adding these two half equations together, we get:
2H2 + 4OH- + O2 + 2H2O + 4e- à 4H2O + 4e- + 4OH-
To get the final equation, we need to cancel out anything that is the same on both sides. In this case, this includes the hydroxide ions, electrons and two water molecules. This results in the following overall equation:
Hydrogen fuel cells are a potential alternative to rechargeable cells and batteries. The advantages of hydrogen fuel cells are that they only produce water as a product (so no toxic products formed). They are also small and easy to maintain. However, they are very expensive to make and require a constant supply of hydrogen gas which is flammable and difficult to store. Non-rechargeable alkaline batteries are cheaper to make but they may accumulate in landfill sites once they have run out. These batteries are recyclable but the recycling process is expensive.
Did you know…
You can make a battery out of potatoes. Scientists have found that boiled potatoes can generate ten times the energy as a raw one. They placed potato slices between a copper cathode and zinc anode and connected them together with wires. The potato battery was able to provide LED lighting for up to 40 days and was sufficient to power mobile phones. It’s suggested that using plant material to generate energy could be useful in developing regions without access to a power grid. Image credit: Mogens Jacobson
Next Page: Rate of Reaction