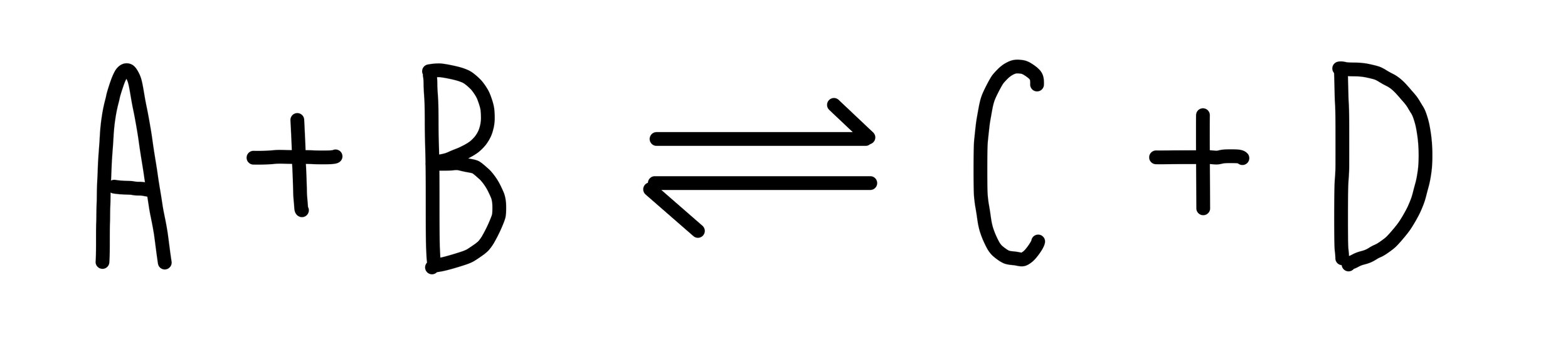Reversible Reactions
Some reactions can go both forwards and backwards - they are reversible. When the forward reaction is happening at the same rate as the backward reaction, it is said to be in dynamic equilibrium.
Reversible reactions
Some chemical reactions are reversible, which means that the products can react with each other to reform the reactants. The reaction is able to go both forwards and backwards. We represent reversible reactions using a double-headed arrow. For example:
The direction of reversible reactions can change depending on the reaction conditions. For example, for the thermal decomposition of ammonium chloride into ammonia and hydrogen chloride, adding heat will cause the forward reaction to happen. Cooling things down will trigger the backwards reaction to happen.
Energy changes and reversible reactions
If a reversible reaction is exothermic (releases heat) in one direction, then it must be endothermic (absorbs heat) in the other direction, and vice versa.
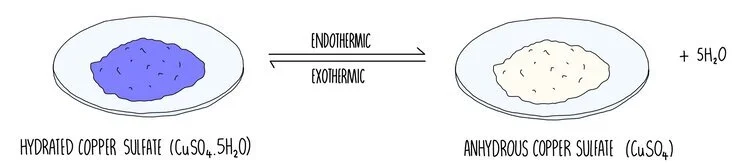
Blue copper sulfate is hydrated which means that the copper and sulfate ions in its crystal structure are surrounded by water molecules. When heated, the water is driven off, producing anhydrous copper sulfate which is a white solid. The reaction is reversible, which means that as soon as any water is around, anhydrous copper sulfate will be converted back into the blue hydrated form. The forward reaction is takes in heat energy (it is endothermic), which means that the reverse reaction must absorb heat energy (exothermic).
Equilibrium
In a closed system (one in which no reactants or products can escape e.g. a sealed container), reversible reactions can reach dynamic equilibrium. Dynamic equilibrium describes how reactants are being formed just as fast as the products are being formed, resulting in the concentrations of the reactants and products staying the same (be careful: this doesn’t mean that the concentrations of reactants and products are equal).
At equilibrium:
The forward and reverse reactions are still happening
The rate of the forward and reverse reactions is the same
The concentration of reactants and products remains constant
Position of equilibrium
When a reaction has reached dynamic equilibrium and a change occurs (such as a change in concentration, pressure or temperature), the position of equilibrium will move to counteract the change. This concept is known as Le Chatelier’s principle.
Concentration
If the concentration of a reactant is increased, the position of equilibrium shifts to the right to favour the formation of products.
Imagine a sealed container filled with ammonium chloride which is decomposing into ammonia and hydrogen chloride. The reaction has reached dynamic equilibrium but then I add more ammonium chloride. The position of equilibrium will then move to the right, to make more products and balance things out. The same is true for the other way round - if I added more product to the container the position of equilibrium would shift to the left to favour the formation of reactants.
Pressure
If the pressure is increased, the position of equilibrium shifts to the side with the fewest moles of gas.
For example, the equation below represents the Haber Process which is an industrial process used to make ammonia for use in fertilisers. From the balanced symbol equation we can see that there are a total of four moles of gas on the left hand side of the equation and only two on the right. If we increase the pressure, the position of equilibrium will shift to the right hand side (towards ammonia) as this is the side with the fewest moles of gas. Alternatively, if we decrease the pressure, the position of equilibrium would shift to the left hand side where there are more moles.
Temperature
If we increase the temperature of a reaction at equilibrium, the position of equilibrium will shift in the endothermic direction to lower the temperature.
For example, hydrogen can be manufactured by reacting carbon with steam, as shown in the equation below. The forward reaction is endothermic and the reverse reaction is endothermic. This means that if we increase the temperature, the position of equilibrium will shift in the endothermic forward direction (towards the products). Alternatively, if we decrease the temperature, the position of equilibrium will shift in the exothermic reverse direction (towards the reactants).
Catalyst
A catalyst does NOT change the position of equilibrium. This is because it speeds up the forward and reverse reactions by the same amount.
Did you know…
Typically glue is either weak and reversible or strong and irreversible, but now scientists have developed a reversible superglue that was made by mimicking the substances found in snail slime. The glue is strong enough to hold a person in place but as soon as water is added to the glue, it softens and becomes less sticky.
Next Page: Crude Oil and Hydrocarbons